Pete Jorgensen
Plant Science & Horticulture
The Biological Effects of Magnetic Fields on Plant Growth
The use of magnetic fields may be unfamiliar territory to many working within standard horticultural areas. The exact mechanisms of magnetic interactions are not yet well understood, but it is unequivocal that organisms from all five kingdoms respond, in some way, to magnetic fields (Buchachenko, 2016; Kishkinev, et al., 2015; Merrill, 2010; Binhi & N, 2002; Edmonds, 2001; L Kirschvink, et al., 1985).
Magnetic fields influence on plant growth
Higher crop yields have been measured from plants grown following magnetic field treatment compared to those which were not including tomato (Jedlička, et al., 2015) (Hozayn, et al., 2016), chick peas and fava beans (Hozayn, et al., 2016).
Increased germination rates and/or speeds have been observed in tomato (De Souza, et al., 2010), asparagus (Soltani, et al., 2006), pea (Iqbal, et al., 2012), sunflower (Vashisth & Nagarajan, 2010), soybeans (Shine, et al., 2011), snow pea and chick pea (Grewal & Maheshwari, 2011). Increased seedling vigour has been reported in soybean (Shine, et al., 2011), sunflower (Vashisth & Nagarajan, 2010), tomato (Jedlička, et al., 2015), snow pea and chick pea (Grewal & Maheshwari, 2011).
These studies show potential for producing crops that mature more quickly, become more robust at an earlier stage and produce higher crop yields. These benefits could become increasingly important if trends towards organic production techniques continue (Reganold & Wachter, 2016; Gay, 2016; Bilalis, et al., 2013; PAN Europe, 2007).
The effect of magnetic fields on plants is still very much an area of nascent development and may be subject to very complex interactions; similar treatments may cause opposite effects depending on field strength and in combination with other variables such as the plant species. The variation of results even within the same study where germination rates were increased, decreased, or not significantly affected for different treatments with no explicable correlation (Gholami, et al., 2010; Martínez, et al.).
Potential explanations of how magnetism affects plant physiology
Proposed explanations include effects on the lipid order of the cell plasma membrane (Poinapen, et al., 2013) , changes to intercellular ion homeostasis and mitochondrial activity (Belyavskaya, 2004), and cryptochrome-induced changes to rates of protein expression (Solov’yov, et al., 2007). The area remains confusing and largely incomprehensible due to much contradictory experimental data and a notable failure of experiments to be consistently reproducible (Belyavskaya, 2004) (Harris, et al., 2009) (Buchachenko, 2016). Examples of poor practice in one study includes the use of only six plants per test group and no attempt to define or quantify the EMF levels being applied as a treatment (Rio & Rio, 2013).
Scientific investigation into the interaction of magnetic fields with plants continues and a recent review by Buchachenko (2016) gives an excellent overview of current idea. One strongly endorsed idea is that magnetic fields affect electron spin, which in turn can influence biochemical reactions; the review provides a thorough explanation of how the comparative low energy of magnetic fields may influence biochemistry. A recent laboratory-based study has shown that chemical reactions involving cryptochrome can be influenced by a magnetic field strength as low as 1mT and with very significant effects at 30mT (Maeda, et al., 2012). There is still need for more research. Some of this will involve subatomic processes, and require high levels of technology and cross-disciplinary collaboration. Definite verifiable explanations are likely to be elusive for some time to come, but this should not prevent further scientific enquiry which may contribute to the body of knowledge relating to the effects of magnetic fields on plants. By way of comparison the mechanisms of photosynthesis have only recently become well understood and explanations about the electron transfer process that are currently taught at graduate level are still uncertain (Peltier, et al., 2016) - this does not prevent enquiry into the effects of light quality on plant growth (Arena, et al., 2016).
Field Strength and Measurement
There are several methods for measuring the strength of magnetic fields. The SI unit for measuring magnetic flux density is the tesla (T); it is also very common for the centimetre–gram–second (CGS) unit - gauss(G) - to be used; 1T = 10,000G (Gregersen, 2011). Another common measurement is the pull force (Fpull) – the amount of force required to pull a magnet free from a flat steel plate. Most studies on the effects of magnetic fields on plants use mT (millitesla) as per table AA2 in the appendix.
It is important to make a distinction between magnetic flux (φ) and magnetic flux density (B). El Taher’s doctoral thesis (El Taher, 2015) measures magnetic flux in the SI unit of webers (Wb) as well as noting the magnetic flux density measured in tesla (1T=1Wb/m2) of the magnets being used. This is important because the use of different sized magnets with equal B gives rise to different levels of φ; it is notable that this distinction is not made in most studies including (Harris, et al., 2009). However, El Taher’s thesis fails to account for the altered field strength caused by the distance of seeds sown from the surface of the magnet: the magnets were in the bottom of the pots and seeds sown towards the top – there is no mention of the height of the pot or the distance between the seed and the surface of the magnet. She has merely calculated the magnetic flux (table 5-1 in the paper) at the surface of the magnet (φ = BA). It would be impossible to replicate this experiment based on the described methods.
A summary of studies is shown in table AA2 in the appendix, including plant types and field strengths (Maffei, 2014). While the range of field strength studied is extensive, and over 25 of the experiments have been carried out using field strengths in the range 1mT-250mT, all have shown significant results. Harris, et al. (2009) did not find significant results using 100mT, but was not included in the table.
Lower field strengths require more sophisticated equipment both in terms of measurements, and shielding (to ensure outside sources / GMF do not influence the experiment). Higher levels above 1T also require pricey equipment, and levels above 2T are currently recognized as harmful to human health (ICNRP, 2009). Use of a static magnetic field around the 100mT is more affordable, measurable and is many orders of magnitude above any magnetic field that would normally be present in the natural environment.
Field Source
Some studies use AC induction coils (Moon & Chung, 2000), others (Aladjadjiyan, 2010) fail to state to the source of the magnetic field, only confirming the strength. Ring magnets were employed by Gholami, et al. (2010) - they fail to state what type of magnet, but do provide the dimensions and B at the magnet surface. Their figures suggest they were using neodymium magnets. However, they have not specified this.
Permanent neodymium magnets in the region of 100mT have been used in experiments on the cryptochrome response of Arabidopsis thaliana to magnetic field (Harris, et al., 2009) and for investigating the effect of magnetic flux on Sorghum bicolor, Zea mays, Eruca sativa, Portulaca oleracea and Corchorus olitorius (El Taher, 2015). They are affordable, convenient, have an excellent field strength to size ratio. Provided they are not subject to excessive environmental or mechanical stress, they can be reused for many years (Jha, 2014).
Exposure Time to Magnetic Field
There is considerable variation in the exposure times used in studies, and it is difficult to find any justification for the times selected. Often different exposure times are used and form an independent variable within the experimental design, but most commonly are between 1 and 15 minutes (see table AA3 in the appendix). Based on these studies, exposure times of 0,1,3 and 5 minutes are suggested.
Magnetic Pole Orientation
The orientation of poles is rarely mentioned in studies. All static magnetic fields have a consistent direction of flow (Getzlaff, 2008). The term ‘pole’ is not mentioned at all in the ‘gold standard’ study by Harris, et al. (2009): this was despite the use of neodymium magnets for part of that study. This lack of information effectively make the experiment unreplicable. One short study into the growth of cress showed a significant difference between growth response for plants treated with a south pole compared to those treated with a north pole (Krótki, et al., 2015). This study used imprecise measurements of comparison, including analysis of the greenness of photographs and diameter of growth area as viewed from above. These are tentative and imprecise measures. Chlorophyll meters were not used; plant weights and other growth indices were not measured. The study also fails to provide any explanation of a ‘synergistic effect with the magnetic field of the planet’; zero references were attached to this statement. The orientation of GMF in the northern hemisphere is south (Shipman, et al., 2016). From reviewing current literature, it is difficult to envisage how pole direction at a level which is several orders of magnitude higher than GMF could influence experimental outcome, but it would still seem pertinent to mention pole orientation within the experimental process.
Magnetism, Water, and Plant Growth
Some experiments treat seeds following imbibition (Martínez, et al., 2014); (Iqbal, et al., 2012); (Aladjadjiyan, 2010); (Gholami, et al., 2010) others during imbibition (Isaac, et al., 2011); (Aksyonov, et al., 2001). A correlation between longer seed exposure to water before treatment has been found (Aksyonov, et al., 2001). Water has been found to reduce surface tension when exposed to magnetic fields (Cai, et al., 2009). Experiments have been carried out on irrigation using magnetized water with significant results as reviewed by Hozayn, et al. (2014). It is therefore proposed that seeds are pre-soaked prior to treatment.
References
Aksyonov, S. I. et al., 2001. Effects of ELF-EMF treatment on wheat seeds at different stages of germination and possible mechanisms of their origin. Electro- and Magnetobiology, 20(2), pp. 231-253.
Aladjadjiyan, A., 2010. Influence of stationary magnetic field on lentil seeds. International Agrophysics, 24(3), pp. 321-324.
Arena, C. et al., 2016. The effect of light quality on growth, photosynthesis, leaf anatomy, and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environmental and Experimental Botany, 130, pp. 122-132.
Belyavskaya, N. A., 2004. Biological effects due to weak magnetic fields on plants. Advances in Space Research, 34(7), pp. 1566-1574.
Bilalis, D. J. et al., 2013. Magnetic field pre-sowing treatment as an organic-friendly technique to promote plant growth and chemical elements accumulation in early stages of cotton. Australian Journal of Crop Science, 7(1), p. 46.
Binhi, V. N., 2002. Magnetobiology: Underlying Physical Problems. 1st ed. London: Academic Press.
Buchachenko, A., 2016. Why magnetic and electromagnetic effects in biology are irreproducible and contradictory? Bioelectromagnetics, 37(1), pp. 1-13.
Cai, R., Yang, H., He, J. & Zhu, W., 2009. The effects of magnetic fields on water molecular hydrogen bonds. Journal of Molecular Structure, 938(1), pp. 15-19.
Cui, L.-L. et al., 2014. Effect of magnetic field on Brassica oleracea L. var. capitata L. seed germination and studies on its mechanism. China Vegetables, 6, p. 10.
De Souza, A., Sueiro, L., García, D. & Porras, E., 2010. Extremely low-frequency non-uniform magnetic fields improve tomato seed germination and early seedling growth. Seed Science and Technology, 38(1), pp. 61-72.
Edmonds, D., 2001. Electricity and Magnetism in Biological Systems. 1st ed. Oxford: Oxford University Press.
El Taher, O. A. M., 2015. Effect of magnetic field on the growth of selected vegetables and cereal crops. Khartoum: (Doctoral dissertation, University of Khartoum).
Evans, M. W. & Cromwell, L. B., 2001. Classical and Quantum Electrodynamics and the (B3) Field. 1st ed. London: World Scientific Publishing.
Gay, I. T., 2016. Trends in pesticide use in agriculture. [Online] Available at: http://biocontroltech.com/2016/02/04/330/ [Accessed 30 March 2017].
Getzlaff, M., 2008. Fundamentals of Magnetism. 1st ed. Berlin: Springer-Verlag.
Gholami, A., Sharafi, S. & Abbasdokht, H., 2010. Effect of magnetic field on seed germination of two wheat cultivars. World Academy of Science, Engineering and Technology, 62, pp. 279-282.
Gregersen, E., 2011. The Britannica Guide to Electricity and Magnetism. 1st ed. New York: Britannica Educational Publishing.
Grewal, H. S. & Maheshwari, B., 2011. Magnetic treatment of irrigation water and snow pea and chickpea seeds enhances early growth and nutrient contents of seedlings. Bioelectromagnetics, 32(1), pp. 58-65.
Hozayn, M. et al., 2016. Applications of magnetic technology in agriculture: A novel tool for improving crop productivity (1): Canola. African Journal of Agricultural Research, 11(5), pp. 441-449.
Hozayn, M., El-Monem, A. A. A., Elwia, T. A. E. & El-Shatar, M. M., 2014. Future of magnetic agriculture in arid and semi-arid regions (case study). Scientific Papers-Series A, Agronomy, 57, pp. 197-204.
ICNRP, 2009. ICNRP guidelines of exposure to static magnetic fields. Health Physics, 96(4), pp. 504-514.
Iqbal, M., Haq, Z., Jamil, Y. & Ahmad, M., 2012. Effect of presowing magnetic treatment on properties of pea. International Agrophysics, 26(1), pp. 25-31.
Iqbal, M.-H. et al., 2016. Pre-sowing seed magnetic field stimulation: A good option to enhance bitter gourd germination, seedling growth, and yield characteristics. Biocatalysis and Agricultural Biotechnology, 5, pp. 30-37.
Isaac, A., Hernández, A., Domínguez, A. & Cruz, O., 2011. Effect of pre-sowing electromagnetic treatment on seed germination and seedling growth in maize (Zea mays L.). Agronomía Colombiana, 29(2), pp. 405-411.
Jedlička, J., Paulen, O. & Ailer, Š., 2015. Research of the effect of low-frequency magnetic field on germination, growth, and fruiting of field tomatoes. Acta Horticulturae et Regiotecturae, 18(1), pp. 1-4.
Jha, A. R., 2014. Rare Earth Materials: Properties and Applications. 1st ed. Boca Raton: CRC Press.
K&J Magnetics Inc., 2017. DY84. [Online] Available at: https://www.kjmagnetics.com/proddetail.asp?prod=DY84 [Accessed 10 March 2017].
Kishkinev, D. et al., 2015. Eurasian reed warblers compensate for virtual magnetic displacement. Current Biology, 25(19), pp. R822-R824.
Knoepfel, H. E., 2000. Magnetic Fields: A Comprehensive Theoretical Treatise for Practical Use. 1st ed. New York: John Wiley & Sons.
Krótki, F. J., Kościelniak, B. K. & Tomasi, P. J., 2015. The influence of strong static magnetic fields on the germination and growth of garden cress (Lepidium sativum L.). American Journal of Experimental Agriculture, 7(4), pp. 251-256.
Kirschvink, J. L., Jones, D. S. & MacFadden, B. J., 1985. Magnetite Biomineralization and Magnetoreception in Organisms. 1st ed. New York: Plenum Press.
LabShop, 2017. 1004-050 50mm diameter Grade Number No. 4 qualitative filter paper circle disc (pack of 100). [Online] Available at: http://www.lab-shop.co.uk/filtration-4593/whatman-qualitative-filter-papers-4479/whatman-diameter-number-qualitative-363798.htm [Accessed 4 April 2017].
Maeda, K. et al., 2012. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proceedings of the National Academy of Sciences, 109(13), pp. 4774-4779.
Maffei, M. E., 2014. Magnetic field effects on plant growth, development, and evolution. Frontiers in Plant Science, 5, p. 445.
Majd, A., Shabrangi, A., Bahar, M. & Abdi, S., 2009. Effect of AC and DC magnetic fields on seed germination and early vegetative growth in Brassica napus L.. Moscow: Progress in Electromagnetics Research Symposium Proceedings.
Merrill, R. T., 2010. Our Magnetic Earth: The Science of Geomagnetism. 1st ed. Chicago: University of Chicago Press.
Moon, J. D. & Chung, H. S., 2000. Acceleration of germination of tomato seed by applying AC electric and magnetic fields. Journal of Electrostatics, 48(2), pp. 103-114.
PAN Europe, 2007. Pesticide Use Reduction Strategies in Europe. London: PAN Europe.
Peltier, G., Aro, E. M. & Shikanai, T., 2016. NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annual Review of Plant Biology, 67, pp. 55-80.
Pietruszewski, S. & Martínez, E., 2015. Magnetic field as a method of improving the quality of sowing material: A review. International Agrophysics, 29(3), pp. 377-389.
Pittman, U. J., 1977. Effects of magnetic seed treatment on yields of barley, wheat, and oats in southern Alberta. Canadian Journal of Plant Science, 57(1), pp. 37-45.
Poinapen, D. et al., 2013. Static magnetic fields enhance lipid order in native plant plasma membranes. Soft Matter, 9(29), pp. 6804-6813.
Reganold, J. P. & Wachter, J. M., 2016. Organic agriculture in the twenty-first century. Nature Plants, 2, p. 15221.
Rio, L. C. & Rio, M. M., 2013. Effect of electro-magnetic field on the growth characteristics of okra (Abelmoschus esculentus), tomato (Solanum lycopersicum), and eggplant (Solanum melongena). International Journal of Scientific and Research Publications, 3(10), pp. 358-367.
Roederer, J. G., 2005. Information and Its Role in Nature. 1st ed. Berlin: Springer.
Shine, M. B., KN, G. & Anand, A., 2011. Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics, 32(6), pp. 474-484.
Shipman, J. T., Wilson, J. D., Higgins, C. A. & Torres, O., 2016. An Introduction to Physical Science. 3rd ed. Boston: Cengage.
Solov’yov, I. A., Chandler, D. E. & Schulten, K., 2007. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophysical Journal, 92(8), pp. 2711-2726.
Soltani, F., Kashi, A. & Arghavani, M., 2006. Effect of magnetic field on Asparagus officinalis L. seed germination and seedling growth. Seed Science and Technology, 34(2), pp. 349-353.
The Editors of Encyclopædia Britannica, 2012. Magnetic field. [Online] Available at: https://www.britannica.com/science/magnetic-field [Accessed 3 March 2017].
Vashisth, A. & Nagarajan, S., 2010. Effects on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to a magnetic field. Journal of Plant Physiology, 167(2), pp. 149-156.
Ziaf, K. et al., 2015. Magnetic field can improve germination potential and early seedling vigor of cabbage seeds. Annual Research & Review in Biology, 6(6), pp. 390-400.
Appendices
Table AA1: Typical magnetic field strengths - adapted from Table 1.1-I in Magnetic Fields: A Comprehensive Theoretical Treatise for Practical Use By Heinz E. Knoepfel (Knoepfel, 2000). * Expected surface field strength (K&J Magnetics Inc).
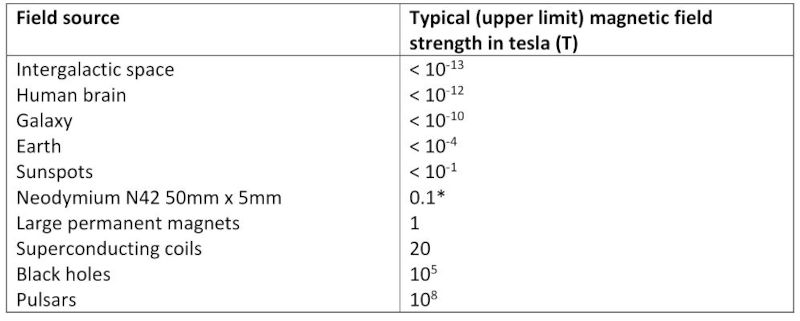
Table AA2: Summary of the effects of magnetic field on plants (Maffei, 2014).
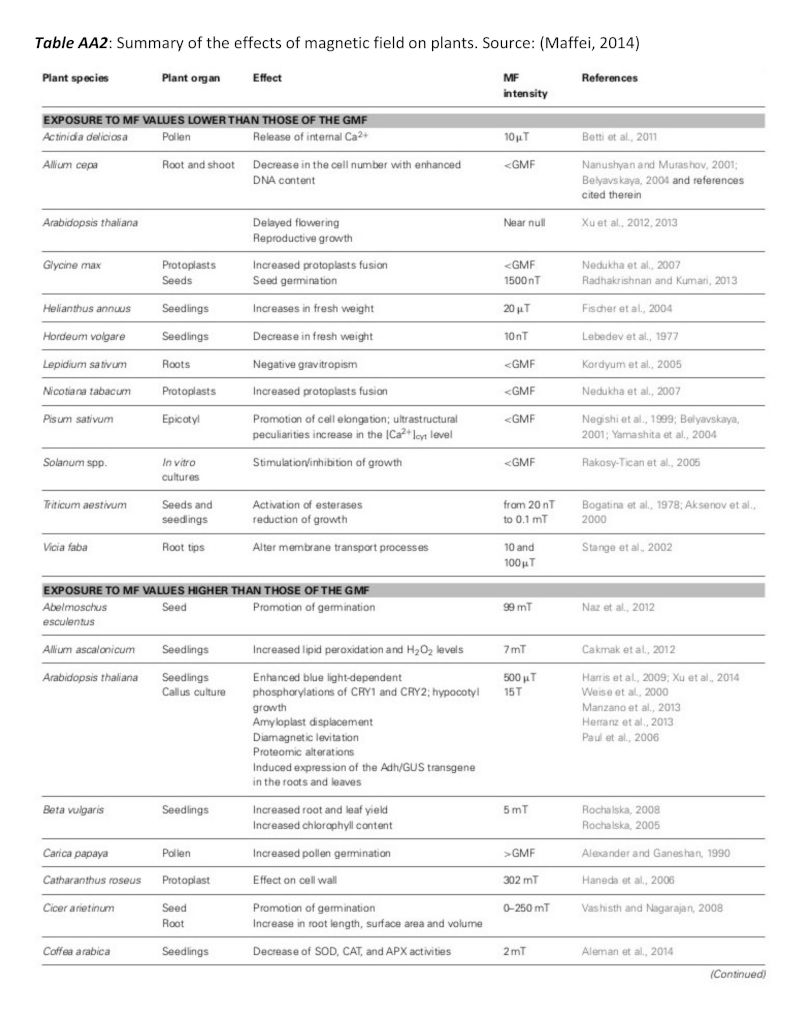
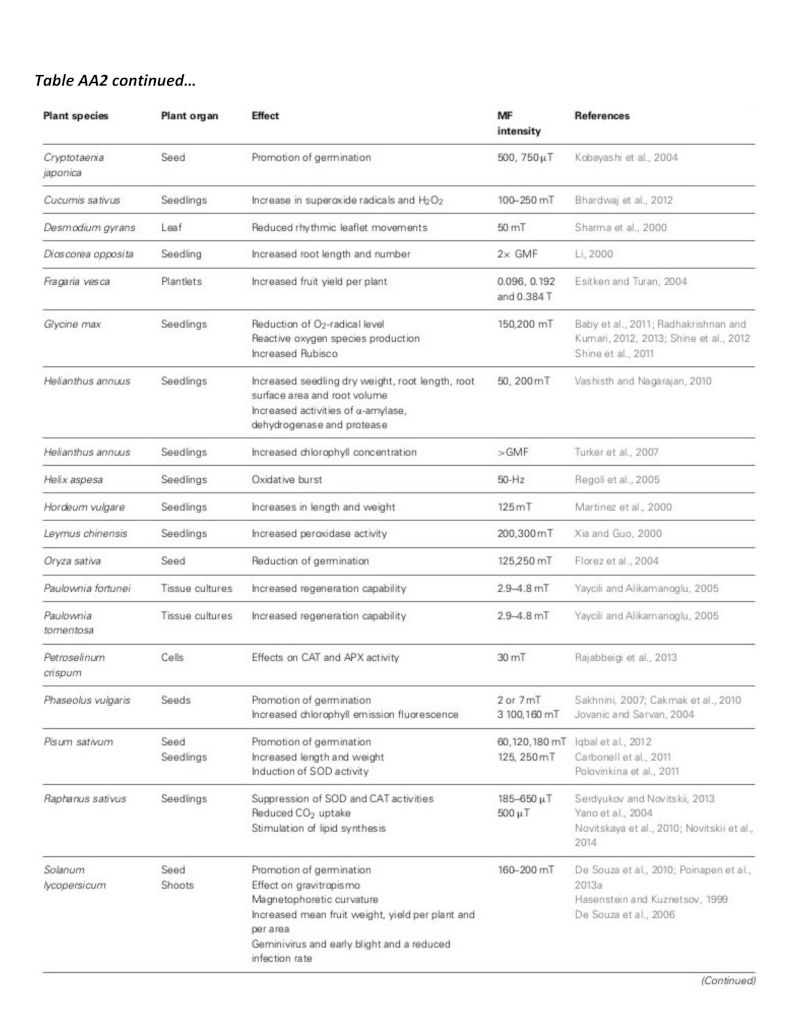
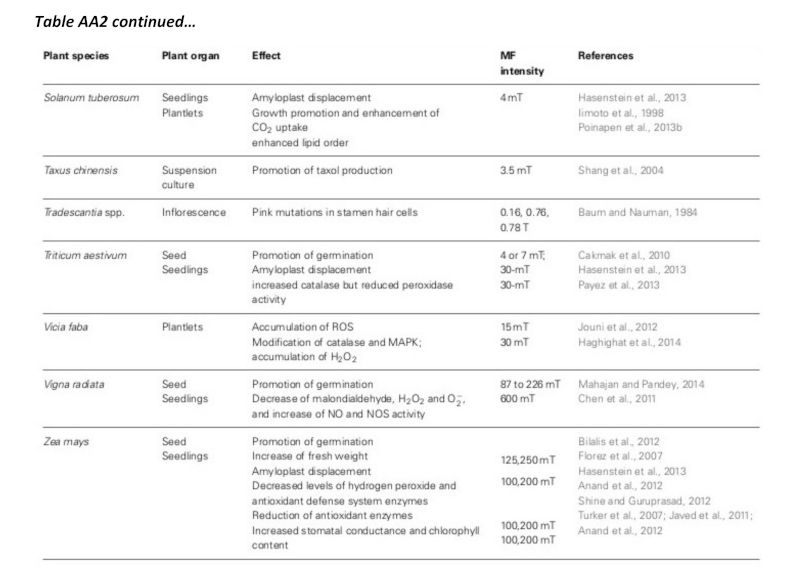
Table AA3: A table of studies using magnetism on plants, showing the exposure times used.
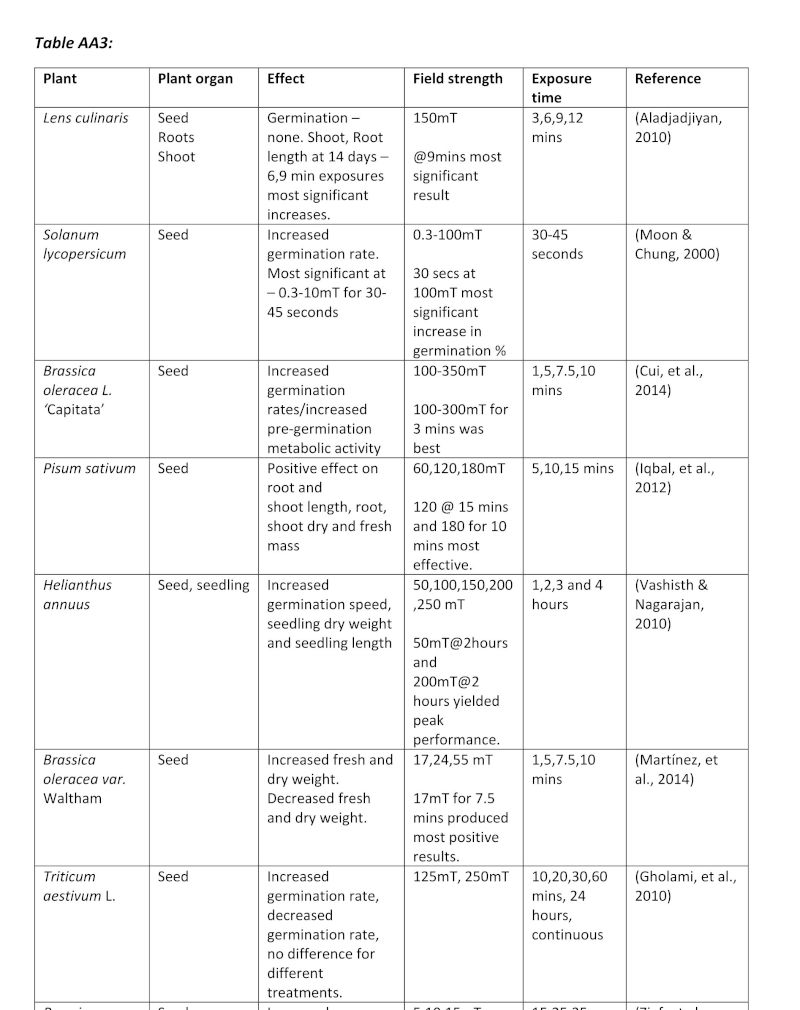
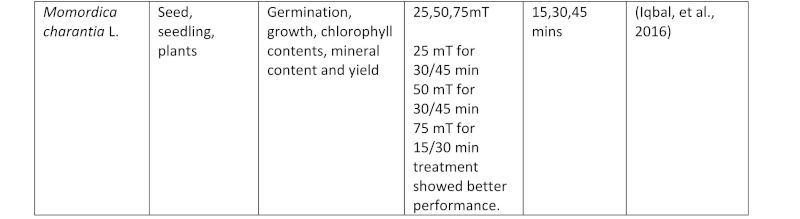
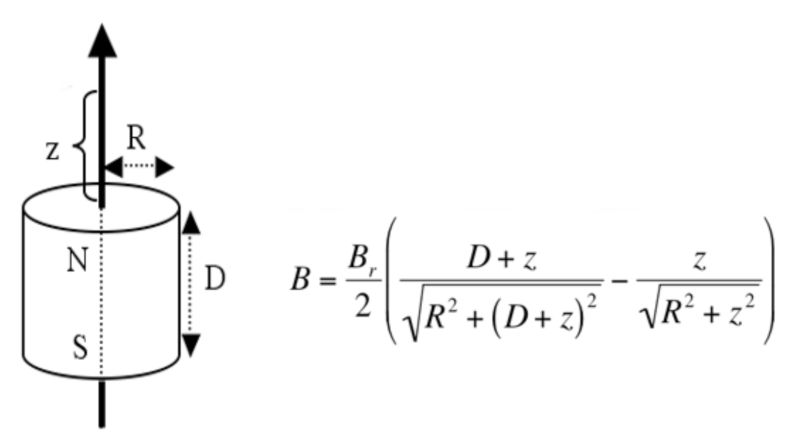
Figure 1A: Equation to calculate the magnetic flux density (B) at a given distance (z) from the pole of a static cylinder magnet with radius R and depth / height D.