Pete Jorgensen
Plant Science & Horticulture
The use of tissue culture in the conservation of Rhododendron sp.
According to the IUCN red list there are currently no fewer than 12 102 threatened plant species worldwide (IUCN, 2017). The genus Rhododendron, a member of the Ericaceae family within the class Magnoliopsida, contains 316 species that have been identified as threatened with extinction in the wild (Gibbs et al., 2011). Many destructive pressures on natural R. habitats are driven by population growth which leads to the expansion of settlements and road networks; overharvesting for firewood, incense and other uses compounds the problem (Singh et al., 2009). The BGCI’s ‘Global Strategy for Plant Conservation 2011-2020’, includes a target (objective II, target 8) for the ex-situ conservation of 75% of threatened species with the aim of rewilding 20% by 2020 (BGCI 1, 2012). A global survey of ex-situ collections found records of a mere 53% of threatened R. and only 48 of the 75 most threatened (BGCI 2, 2012). There is an urgent need to increase the number of threatened R. species in ex-situ collections.
Detailed below, is a trial of methods for the micropropagation of three R. species including R. seinghkuense aff., a plant identified as significantly similar to R. seinghkuense which is threatened with extinction in the wild (Gibbs et al., 2011). Different strengths of Anderson’s Rhododendron Media + vitamins (ARM+), with the addition of plant growth hormones in varying concentrations, prove to be a successful basis for the propagation of R. floral bud explants and seeds. A protocol for the micropropagation of R. sp. seeds is proposed, although further studies are recommended to verify its efficacy.
Background
Rhododendron can be a costly and invasive pest in the UK, covering almost 100,000 hectares of land, and damaging ecosystems and destroying biodiversity (McEwan, 2016). R. ponticum is often identified as the predominant pest species. However, problematic invasive plants are often hybrids (Forest Research, 2017; Woodland Trust, 2013). The cost of removing R. from the Trossachs national park in Scotland is estimated to be £25 million, although clearance costs are often mitigated by using unpaid voluntary labour (RAFTS, 2017; Pimentel, 2002).
Considering these issues, it is understandable why some may view R. as unworthy of the expense of conservation, however, a full and balanced consideration of this often breathtakingly beautiful genus provides a different perspective. It is the national flower of Nepal and has immense cultural and economic value across the globe. Uses include: timber, firewood, wine, honey, tea, jam, biocide and medicine (BGCI, 2011). Some R. are edible and others, including the maligned R. ponticum, contain compounds that may be useful in the treatment of Alzheimer’s disease (Singh et al., 2009; Orhan et al., 2004).
The UK hosts the world’s two largest ex-situ collections of R., one at the Royal Botanic Garden Edinburgh, the other at the Royal Botanic Gardens, Kew (BGCI 2, 2012). An eponymous national collection of R. that includes many rare and unique plants, is held at The Lost Gardens of Heligan - a popular tourist destination in SW England (Heligan, 2015). Heligan’s plants suffered from a serious Phytophthora problem, which gave rise to a micropropagation conservation program that commenced in 2008 (Heligan, 2015). The work was successful and resulted in many disease-free plants being produced to replace or add to those threatened and destroyed by disease (Heligan, 2015).
Tissue culture is an effective means of propagating plants for conservation purposes; it is particularly useful where germplasm is rare, when traditional cultivation techniques are slow or unreliable, and/or when it is desirable to create multiple plants from a single source (Fay, 1992). The attempted micropropagation of three species of R. are the subject of this report with the aim of developing protocols that may be deployed in future conservation programmes. The first, R. dalhousieae Hook. f. (R. dalhousieae var. dalhousieae is a synonym), was propagated from floral bud explants (The Plant List 1, 2012). The second, R. maddenii Hook. f (R. maddenii spp. maddenii is a synonym), was propagated from seed (The Plant List 2, 2012). The third, also propagated from seed, was R. seinghkuense aff. – potentially a new species or sub-species that has a close morphological resemblance to R. seinghkuense Kingdon-Ward ex Hutch. but may not be identical (The Plant List 3, 2012). Example inflorescences, key botanical information and endemic geographical locale are presented in appendix 1.
Methods
Anderson’s Rhododendron Media with vitamins (ARM+), adjusted to pH 5.7, was used as the basal media in all experiments. In some cases, the medium was diluted to a lower strength: table 1 indicates the concentration of the basal medium and table 2 the hormone levels - a list of ingredients and proportions used in full-strength ARM can be viewed in appendix 4.
Table 1: The strength of ARM+ (Anderson’s Rhododendron Media with vitamins) used in each media.
Media |
ATZ |
A1 |
A4 |
A16 |
Strength |
¼ |
Full |
½ |
Full |
Table 2: Strength of hormones used in each media in mg/L.
Hormone |
Hormone full name |
ATZ |
A1 |
A4 |
A16 |
2iP |
N6-(Î2-isopentenyl)-adenine |
5 |
5 |
5 |
None |
IAA |
Indole-3-acetic acid |
1.9 |
1.9 |
1 |
None |
TDZ |
Thidiazuron |
1 |
None |
None |
None |
R. dalhousieae - floral buds
Floral buds were prepared, sterilized and inoculated into ATZ media per the procedure in appendix 1 (day 0). They were then placed into a controlled environment of 25°C +/- 1°C under white fluorescent light 30μmol m−2 s−1 (+/- 1) with a 16-hour photoperiod. After 5 weeks (day 35) they were transplanted into A4 media and observations made after a further 4 weeks (day 63), 10 weeks (day 105), 13 weeks (day 126) and 17 weeks (day 154). On day 105, due to contamination, the buds were cleaned, dissected and transferred to fresh A4 media - the worst affected parts were disposed of and 5 pieces, each with a basal section consisting of approximately 2-3mm callus material, were re-inoculated.
R. seinghkuense .aff and R. maddenii - seeds
Seeds were prepared, sterilized, and inoculated onto A16 media per the procedure in appendix 2 (day 0). Following this they were placed in a controlled environment of 25°C +/- 1°C with 16-hour photoperiod under white fluorescent light - 30μmol m−2 s−1 (+/- 1). After 4 weeks (day 28) R. maddenii seeds were disposed of due to contamination (sterilized prior to disposal by autoclaving at 121°C for 15 minutes. At the same stage some R. seinghkuense seedlings had emerged and these were separated into two groups – one seedling was moved into fresh A16 media, another three were placed into A1 media. The ungerminated R. seinghkuense seedlings were kept in the A16 media. After a further 5 weeks (day 70) observations were made and the seedlings in the A1 media were transplanted into compost. Observations were taken after a further 3 weeks (day 91) at which point the ungerminated batch of seedlings in the A16 media was disposed of (following sterilization). Further observations were made after an additional 4 weeks (day 119).
Results
R. dalhousieae - floral buds
The following documentation of the progress of the development of the floral buds is accompanied by a photographic record – figure 1.
DAY 35 (8th Nov 2017)/ATZ: Figure 1-A, all buds had grown in size, slightly pale green and fleshy, a small amount of browning/necrosis. Signs of contamination - a white milky haze in the gel surrounding the inoculation sites. No root development.

Figure 1: The progress of R. dalhousieae floral buds in tissue culture. (A) Day 35 in ATZ medium, (B) Day 63 in A4 medium, (C) Day 105 in A4, (D) Day 126 in A4, (E) Day 126 in fresh A4 after being cleaned / dissected, (F) Day 154.
DAY 63 (6th Dec 2017)/A4: Figure 1-B, increased browning/necrosis. Continued growth of fleshy leaf/stem type tissue. Signs of callus formation around the top of the pedicel. Contamination now spread in a filamentous pattern from the locus of inoculation outwards to edge of container. No root development.
DAY 105 (17th Jan 2018)/A4: Figure 1-C, buds now approximately double their initial size, pale green stem/leaf type tissue, increased signs of browning/necrosis. Callus development more pronounced, approximately 3 to 4 mm in diameter. Contamination still evident. No root development.
DAY 126 (7th Feb 2018)/A4: Figure 1-D, no significant additional growth. Slightly paler green, increasingly blanched. Contamination worse, spreading outwards. Basal sections in contact with media beginning to brown. No root development. Explants removed, cleaned, dissected and re-inoculated into fresh media Figure 1-E.
DAY 154 (7th Mar 2018)/A4: Figure 1-F, cleaned bud sections had developed new growth - darker green, healthier, and more differentiated than initial bud development. Slight signs of contamination. No root development.
Only one media was used so no statistical comparison is possible. Contamination reduced the number of buds involved from 7 to 4 after 35 days, and explants were cleaned and dissected on day 105.
R. seinghkuense .aff - seeds
The following description of the progress of the seedling growth is accompanied by a photographic record (figure 2).
DAY 28 (6th Dec 2017) A16: Figure 2-A, 4 seeds out of the 25 germinated and showing young growth – green shoots 2-3mm each with small leaves approximately 2mm long and 1mm wide. No contamination was evident. No root development. One seedling moved to A1 media (Figure 2-E).
DAY 70 (17th Jan 2018) A1/A16: No additional germination. The three seedlings left in the A16 gel (Figure 2-B) have between 5 and 6 leaves each (5, 6 and 6) and grown to around 10mm in height with 3-4mm of root present - moved to compost. The seedling in A1 gel (Figure 2-F): 18 tiny stems <5mm in length with one tiny leaf per stem.
DAY 91 (7th Feb 2018) A1/A16/compost: No additional germination. Three seedlings in compost (Figure 2-C) had elongated to 20mm, leaves increased in area but no new leaves. Seedling in A1 (Figure 2-G) no further elongation, still <5mm in length. Shoot proliferation - estimated to be >25 individual stems, no roots.
DAY 119 (7th March 2018) A1/compost: Three seedlings in compost - taller, approximately 25mm, larger leaves (Figure 2-D). Hairs visible on both the stem and leaves. Additional two leaves on one of the stems giving it a total of 8, the other two still had 5 and 6 leaves. Roots thickened. Seedling in A1 (Figure 2-D) - many more stems, now estimated at >35, and the overall height taller - approximately 10mm, no roots.
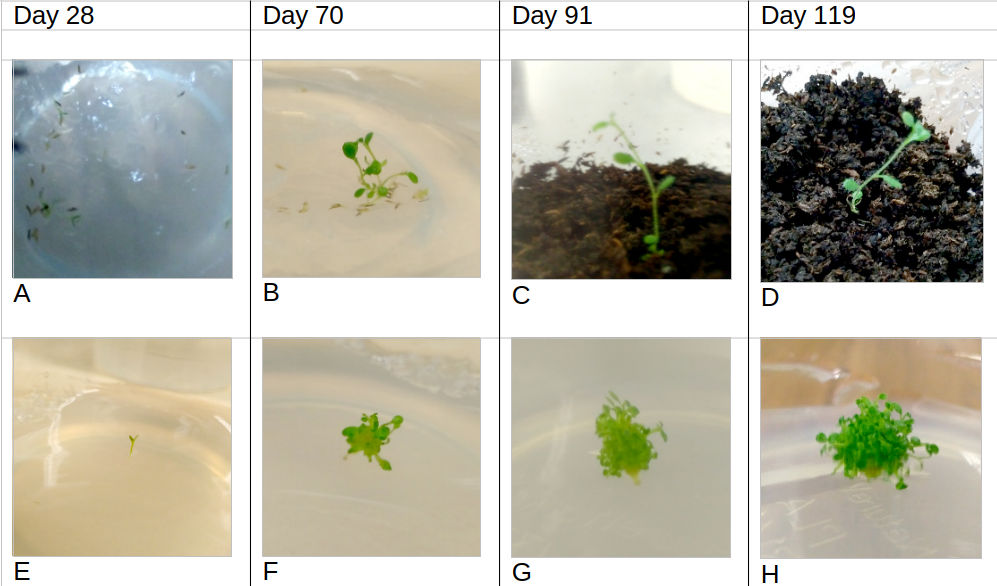
Figure 2: The progress of R. seinghkuense aff. seeds in tissue culture. (A) Day 28, four seeds germinated. (E) Day 28 one seedling transplanted to A1 media. (B) Day 70 – seedlings in A16 media prior to being transplanted into compost. (F) Day 70 – seedling in A1 media. (C) Day 91 example seedling in compost. (G) Day 91, seedling in A1 media. (D) Day 119, example seedling in compost. (H) Day 119, seedling in A1 media. Germination rate: 4 seeds germinated out of 25 within 28 days (16%) with no further germination occurring after this. Only one variable was used (media=A16) so there are no meaningful ways of analysing this data further.
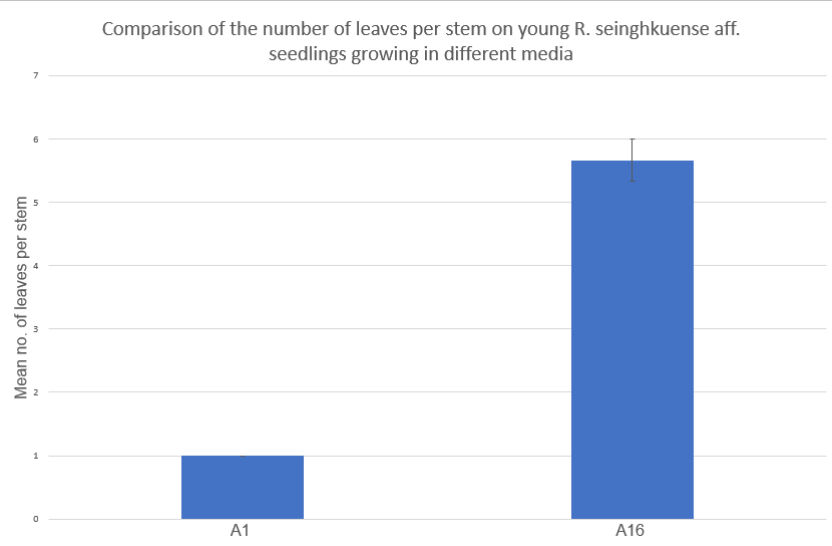
Figure 3: Comparison of the mean number of leaves per stem on tissue culture explants growing in two different media A1 and A16, 70 days following the sowing of seed. Error bar included (standard error of the mean) – all stems in group A1 had one leaf so there is no deviation to display.
Seedling development: between day 28 and day 70 the germinated seeds were separated into two groups. Group 1 in A1 (n=1), group 2 in A16 (n=3). The seed in the A1 group developed 18 stems by day 70 (42 days from transference) each with one leaf, the three in the A16 group remained a single stem with a mean number of 5.66 leaves per stem – see figure 3. Further statistical analysis would only be meaningful with a greater number of replicates.
R. maddenii - seeds
DAY 28 (6th Dec 2017): The plate had severe contamination - a fuzzy grey/white covering across whole of gel around 1cm deep; the gel also had a brown tinge. It was not possible to make observations of the seeds and the unit was disposed of after being sterilised.
Discussion
R. dalhousieae - floral buds
The addition of the cytokinins TDZ and 2iP, and the auxin indole-3-acetic acid (IAA) has been found to increase shoot proliferation in Rhododendron bud explants consisting of ovary and pedicel tissue (Tomsone et al., 2004). Tomsone found that increasing concentrations of TDZ correlated with increased shoot proliferation but also caused a trade-off in decreased shoot length. The ideal range in that study was 0.5 mg/l to 1mg/l TDZ – figure 4 - but the media also contained 15 mg/L of the cytokinin N6-(Δ2-isopentenyl)-adenine (2iP) and 3 mg/L of the auxin indole-3-butyric acid (IBA).
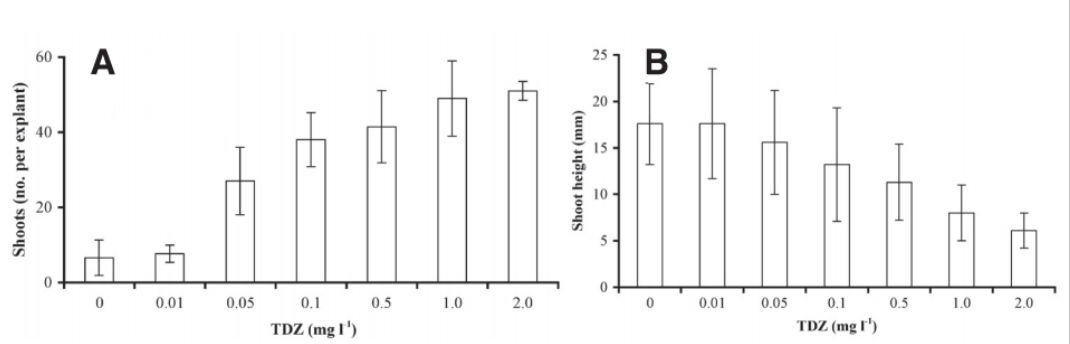
Figure 4: The effect of thidiazuron (TDZ) on shoot regeneration from flower explants of Rhododendron cv. 'Irina'. A, shoot number; B, shoot height. Source: (Tomsone et al., 2004).
In this study R. dalhousieae was first inoculated into ATZ media containing 1mg/L TDZ, 1.9mg/L IAA and 5mg/L 2iP. The buds appeared to increase their mass although no empirical results were obtained. The tissue type that developed was predominantly green and leaf-like. The gel became contaminated early on which could affect the development of the plant tissue (George et al., 2008). Establishing a contaminant-free explant in growing media is often the most difficult aspect of tissue culture (Einset, 1986).
When the buds were moved into A4 media which contained the same amount of 2iP, no TDZ and lower IAA, there was clear and continued callus formation. A balance of auxin and cytokinin can prevent signal transduction pathways from providing clear transcriptional signals to cells, resulting in an undifferentiated cluster of embryonic plant tissue (George et al., 2008). The exact balance can be variant specific. For R. dalhousieae A4 media (5mg/L 2iP and 1mg/L IAA) was effective in producing callus. However, endogenous hormone production may also be a significant factor (Brand et al., 2000).
The smaller, cleaner, explant material inoculated into fresh A4 media on day 126, produced secondary embryogenesis leading to the organogenesis of stem/leaf material without callus formation. It appears that the original floral bud plant tissue had a different response than the dissected callus material to the A4 media. It may be that the cultivated plant cells developed subtle differences in epigenetic response to the PGRs, or the contaminant, which appeared bacterial, may have interfered with the uptake of hormones or activated defence responses which affected cellular processes (Us-Camas et al., 2014).
Both the production of callus or stem material can be useful. Stem material can be nurtured and then transplanted into a root inducing media (with higher auxin and/or lower cytokinin levels) with the aim of producing an entire plant to transplant into compost and harden off. Callus material can be cultured, divided and inoculated into media for organogenesis too, it has potential for producing a larger number of plants. The disadvantage is that genetic mutation is more probable with each iteration of sub-culture (Us-Camas et al., 2014).
R. maddenii and R. seinghkuense seeds
The R. maddenii seeds were lost due to contamination, but (Singh et al., 2009) observed germination of the species within 3 weeks on hormone-free media. Similar results for R. seinghkuense seeds were obtained here: hormone-free media, combined with exposure to light and constant temperature (25°C), induced germination, and produced healthy seedlings. The germination rate of 16% appears low. A search of the literature revealed no comparable studies for R. seinghkuense, but a study that measured germination rates for R. ponticum found none lower than 70% (Erfmeier & Bruelheide, 2005). Cursory examination using a microscope revealed no obvious issues with viability, but the seeds used for this study were collected in the wild in 2012, transported some 6 000 miles and stored in cool ambient conditions for several years - low germination rates would be expected. Additional experimentation would be required to consider the comparative efficacy of the methods used; media composition, light levels, temperature, humidity and altitude may all be influential factors.
Multiple stem proliferation was induced in R. seinghkuense seedlings using A1 media (5mg/L 2iP + 1.9 mg/L IAA). 2iP is recommended as a more effective cytokinin for inducing shoot proliferation than BA, and it was evidently effective here (Singh et al., 2009). Singh et al. (2009) found increased 2iP (7mg/L) and lower IAA (0.1 mg/L) were more effective in terms of stem proliferation with R. maddenii, however, the maximum number of stems counted was 12 – a lower number than observed in this study. It is difficult to know if these differences are solely the result of hormone levels. Singh et al. (2009) also used a modified AM with a different nutrient balance, for example 440 mg/l CaCl2.6H2O and 380 mg/L NaH2PO4 compared to 332.2 mg/l and 380 mg/L used here; there were other nutrient and vitamin differences and a lower temperature of 17°C +/- 1°C was used by Singh et al. (2009). In another experiment, Singh et al. (2003) found that the use of a filter paper bridge and a liquid media could produce 10x the shoot proliferation of a solidified gel media. However, cultivating 18 stems within 70 days is a positive outcome; on that basis further division and subculture could potentially produce over 5 000 explants within 210 days - a useful means of mass production, although there would be some risk of genetic mutation (Smulders & De Klerk, 2011).
The A16 media (no hormones) produced a more natural single-stemmed seedling with small roots - growth was influenced only by endogenous hormones. For larger scale propagation, explants could be multiplied using A1 media and then individual nodes transplanted into A16 media to produce whole individual plants with roots. Following this, a sterile compost can be used to grow seedlings into larger young plants before gradual acclimation in normal garden soil to prepare for outdoor planting.
Conclusion
R. sp. was successfully propagated in Anderson’s medium, replicating past successes (Singh et al., 2003; Eeckhaut et al., 2010; Gurung & Singh, 2010). Callus can be produced from R. dalhousieae floral buds using half strength ARM+ with 5 mg/L 2iP and 1mg/L IAA. Stem and leaf organogenesis was observed after the re-inoculation of dissected callus material into the same media. Further studies would be required to confirm the reliability of this technique for this species. R. seinghkuense seeds that have been in storage for several years can be germinated successfully on full-strength, hormone-free, ARM+ media, albeit with low rates (16%). Stem proliferation can be induced by inoculating seedlings into ARM+ media containing 5mg/L 2iP + 1.9 mg/L IAA. Hormone-free ARM+ media can be used to create single-stemmed seedlings with leaves and roots. These techniques could be combined as part of a conservation program to produce multiple plants from a single seedling. Maturation and acclimation could follow a program similar to one described by Singh & Gurung (2009). Appendix 5 contains a proposed protocol for the propagation of R. seinghkuense from seed to young plant, this could also form the basis of a protocol for other R. sp. Further experimentation with a larger number of replicates would be required to ensure that the results obtained here can be reliably reproduced, and possibly to trail modifications to the process.
References
BGCI, 2011. A Quarter of Rhododendron Species Under Threat. [Online]
Available at: http://www.bgci.org/news-and-events/news/0844/?sec=resources&option=com_news&id=0844
[Accessed 19 October 2017].
BGCI 1, 2012. The Global Strategy for Plant Conservation: 2011-2020, Richmond: Botanic Gardens Conservation International.
BGCI 2, 2012. Global Survey of Ex situ Rhododendron Collections. [Online]
Available at: https://www.bgci.org/files/Worldwide/Conservation/global_survey_of_ex_situ_rhododendron_collections.pdf
[Accessed 18 04 2018].
Brand, M. H., Ruan, Y. & Kiyomoto, R., 2000. Response of Rhododendron Montego'with “Tissue Proliferation” to Cytokinin and Auxin In Vitro.. HortScience, 35(1), pp. 136-140.
Eeckhaut, T., Janssens, K., De Keyser, E. & De Riek, J., 2010. Micropropagation of rhododendron. In: Protocols for in vitro propagation of ornamental plants. New York: Humana Press, pp. 141-152.
Einset, J. W., 1986. A practical guide to woody plant micropropagation. Arnoldia, 46(1), pp. 36-44.
Erfmeier, A. & Bruelheide, H., 2005. Invasive and native Rhododendron ponticum populations: is there evidence for genotypic differences in germination and growth?. Ecography, 28(4), pp. 417-428.
Fay, M. F., 1992. Conservation of rare and endangered plants using in vitro methods. In Vitro Cellular and Developmental Biology, 28P(1), pp. 1-4.
Forest Research, 2017. Rhododendron control. [Online]
Available at: https://www.forestry.gov.uk/fr/rhododendroncontrol
[Accessed 19 October 2017].
George, E. F., Hall, M. A. & De Klerk, G. J., 2008. Plant growth regulators II: cytokinins, their analogues and antagonists. In: Plant propagation by tissue culture. Dordrecht: Springer, pp. 205-226.
Gibbs, D., Chamberlain, D. & Argent, G., 2011. The red list of rhododendrons. Botanic Gardens Conservation International.
Gurung, B. & Singh, K.K., 2010. In vitro mass propagation of Sikkim Himalayan Rhododendron (R. dalhousiae Hook. f.) from nodal segment. The Society for the Advancement of Horticulture, 12(1), pp. 42-45.
Heligan, 2015. The Lost Rhododendrons Return: The Micropropagation Project. [Online]
Available at: http://heligan.com/explore/news/the-lost-rhododendrons-return-the-micropropagation-project/
[Accessed 10 April 2018].
IUCN, 2017. Table 3b: Status category summary by major taxonomic group (plants). [Online]
Available at: http://cmsdocs.s3.amazonaws.com/summarystats/2017-2_Summary_Stats_Page_Documents/2017_2_RL_Stats_Table_3b.pdf
[Accessed 19 10 2017].
McEwan, G., 2016. Rhododendron in UK covers nearly 100,000 hectares. [Online]
Available at: https://www.hortweek.com/rhododendron-uk-covers-nearly-100000-hectares/arboriculture/article/1406788
[Accessed 19 October 2017].
Orhan, I., Sener, B., Choudhary, M. I. & Khalid, A., 2004. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. Journal of Ethnopharmacology, Issue 91, pp. 57-60.
Pimentel, D., 2002. Biological Invasions. 1st ed. London: CRC Press.
RAFTS, 2017. RAFTS Invasive Species Program. [Online]
Available at: http://www.invasivespeciesscotland.org.uk/invasive-species/
[Accessed 19 October 2017].
Singh, K. K. & Gurung, B., 2009. In vitro propagation of R. maddeni Hook. F. an endangered Rhododendron species of Sikkim Himalaya. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 37(1), pp. 79-83.
Singh, K. K., Kumar, S., Rai, L. K. & Krishna, A. P., 2003. Rhododendrons conservation in the Sikkim Himalaya. Current Science, 85(5), pp. 602-606.
Singh, K. K., Rai, L. K. & Gurung, B., 2009. Conservation of Rhododendrons in Sikkim Himalaya: An Overview. World Journal of Agricultural Sciences, 5(3), pp. 284-296.
Smulders, M. J. M. & De Klerk, G. J., 2011. Epigenetics in plant tissue culture. Plant growth regulation, 63(2), pp. 137-146.
The Plant List 1, 2012. Rhododendron dalhousieae var. dalhousieae. [Online]
Available at: http://www.theplantlist.org/tpl1.1/record/tro-12303788
[Accessed 06 April 2018].
The Plant List 2, 2012. Rhododendron maddenii subsp. maddenii. [Online]
Available at: http://www.theplantlist.org/tpl1.1/record/tro-12303781
[Accessed 04 April 2018].
The Plant List 3, 2012. Rhododendron seinghkuense Kingdon-Ward ex Hutch.. [Online]
Available at: http://www.theplantlist.org/tpl1.1/record/tro-50279932
[Accessed 5 April 2018].
Tomsone, S., Gertnere, D. & Novikova, D., 2004. The influence of thidiazuron on shoot regeneration and proliferation of rhododendrons in vitro. Acta Universitatis Latviensis ser. Biology, Issue 676, pp. 239-242.
Us-Camas, R., Rivera-Solís, G., Duarte-Aké, F. & De-la-Pena, C., 2014. In vitro culture: an epigenetic challenge for plants. Plant Cell, Tissue and Organ Culture, 118(2), pp. 187-201.
Woodland Trust, 2013. Woodwise: Invasive species management in woodland habitats., Grantham: Woodland Trust.
Appendices
Appendix 1: Key facts pertaining to the taxa that are subject of this report.
Table A1(1): Inflorescences from left to right: R. dalhousieae, R. maddenii, and R. seinghkuense.

(Hootman, 2004)

(Hirsutum, 2009)

(Rhododendronforeningen, 2012)
Table A1(2): Key characteristics for R. dalhousieae var. dalhousieae, R. maddenii ssp. maddenii and R. seinghkuense sp. – (McQuire & Robinson, 2009; Paul et al., 2005; Flora of China, 2018; Gibbs et al., 2011; Hirsutum, 2018; ARS, 2018).
Species |
R. dalhousieae var. dalhousieae |
R. maddenii ssp. maddenii |
R. seinghkuense |
Habit |
Sprawling shrub to 2 metres. |
Shrub 1-3 metres high, smooth brown flaky bark, branchlets may be scaly |
Shrub 0.3-1 metres high, branches very woolly, may be scaly |
Flowers |
May-July 2-6 fragrant flowers per inflorescence, cream, yellowish inside |
May-July 2-6 flowers per inflorescence, corolla 6.5-10cm long, fragrant, densely scaly outside, white/pinkish, pink blotch |
April-May Usually 1 (rarely 2) sulphur yellow, corolla 2-2.5cm long, dense scales on outer. |
Leaves |
Oblong ovate to 17cm in length |
5.3-14.5cm long, approx. 1/3 as broad, lanceolate, upper surface dark green, underside very scaly |
Lanceolate 4-8cm long and half as wide, bullate glabrous upper, underside very scaly â small brown scales |
Habitat |
E. Nepal, Sikkim, West Bengal 2000-2650 metres altitude |
Sikkim Himalaya 1500-2750 metres altitude |
Seinghku Valley, Burma, NW Yunnan. 1850-3050 metres altitude |
Conservation status |
IUCN â not yet assessed. |
Rare. IUCN â least concern (LC). |
IUCN â threatened with extinction in the wild. Vulnerable (VU). |
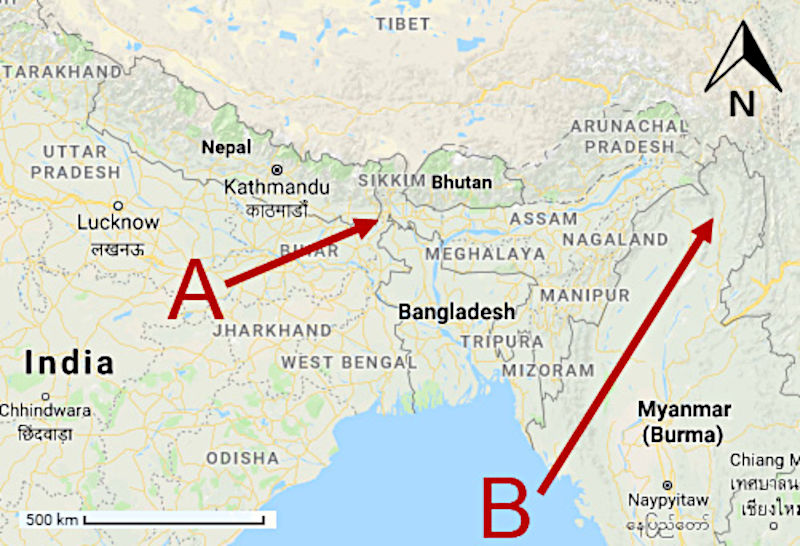
Figure A1(3): A map showing the geographical origins of (A) R. dalhousieae var. dalhousieae and R. maddenii ssp. maddenii, and (B) R. seinghkuense aff. Adapted from Google Maps, 2018.
Appendix 2: Preparation of floral buds for micropropagation
- Wear lab coat, protective gloves, and eye protection.
- Take the bud and using a strong blade carefully remove as many of the outer scales as possible (until the outline of the floral buds can be seen through the scales).
- Brush the bud gently in the direction of the tip with a toothbrush that has been dipped in 30% bleach (domestos) solution.
- Rinse the bud using distilled water and place on a clean paper towel.
- Cut off any excess stalk leaving approximately 5mm of stalk attached the bud.
- Place the bud in a clean plastic cup and cover with 30% bleach (domestos) solution.
- Leave for 20 minutes – agitate periodically.
- Take the bud in the bleach solution to the laminar flow cabinet.
- Drain the bleach solution and rinse twice in sterile distilled water.
- Add a third rinse and leave to stand for 5 minutes before draining.
- Move the bud to a sterile tile and using sterile scalpel and tweezers remove the remaining scales to expose any florets present.
- Florets should be removed with a small length of pedicel attached and placed into an anti-oxidant (ascorbic 200mg/L and citric acid 150mg/L) + TDZ soak for 10 minutes.
- Once all florets are removed and have been soaked in anti-oxidant for 10 minutes they should then be inoculated into containers with ATZ gel up to a maximum of five per pot.
- Lids should be replaced on the pots as soon as possible and then labelled with the plant name, date and your initials.
- Inoculated, sealed, and labeled pots should then be moved to the grow room.
Appendix 3: Seed preparation, sterilisation, and inoculation procedure.
- Wear lab coat, protective gloves and eye protection.
- Remove seeds/seed pod from packet onto a clean piece of paper.
- Using a knife blade or needle carefully extract seeds from seed pods (if required).
- Separate out around 30 viable seeds.
- Dissect a circular filter paper with scissors and fold into a parcel.
- Open the parcel up, add seeds carefully using tweezers and fold up again. Avoid adding any unwanted plant material i.e. seed pod. Secure the seed parcel closed using a paper clip.
- Add seeds to a clean lidded plastic cup and clearly label with the plant ID number.
- Make up 100ml of 20% bleach (domestos) solution using distilled water.
- Pour bleach solution into the plastic cup with the seed parcel, add a stir bar, replace lid and put onto a stirring machine for 20 minutes.
- Remove the plastic cup from the stirring machine and move to a laminar flow cabinet. Drain the contents and rinse twice with sterile distilled water. Add a third rinse leave to stand for 15 minutes.
- While waiting ensure the work area is clean – use a cotton swab and alcohol.
- Drain the plastic cup of the third rinse and using tweezers remove the seed parcel and place on work tile.
- Open out the seed parcel as flat as possible with losing the contents.
- Carefully open a pot of growing media and using tweezers place the opened out seed parcel filter paper upside down onto it so as to inoculate the seeds into the jelly. Press down gently.
- Remove the filter paper and check that all seeds are removed from the paper and are present in the jelly. Use tweezers to move any stray seeds from the paper to the jelly.
- Replace the lid on the growing media pot and label with the plant ID, the date, and responsible party.
Appendix 4
Table A4(1): The concentration of ingredients used in Anderson’s Rhododendron Media with vitamins (ARM+).
Micro Elements |
Mg/L |
µM |
CoCl2.6H2O |
0.025 |
0.11 |
CuSO4.5H2O |
0.025 |
0.10 |
FeNaEDTA |
73.40 |
200.00 |
H3BO3 |
6.20 |
100.27 |
KL |
0.30 |
1.81 |
MnSO4H2O |
16.90 |
100.00 |
Na2MoO42H2O |
0.25 |
1.03 |
ZnSO47H2O |
8.60 |
29.91 |
Macro Elements |
Mg/L |
µM |
CaCl2 |
332.02 |
2.99 |
KNO3 |
480.00 |
4.75 |
MgSO4 |
180.54 |
1.50 |
NaH2PO4 |
330.60 |
2.75 |
NH4NO3 |
400.00 |
5.00 |
Vitamins |
Mg/L |
µM |
Adenine sulphate |
80.00 |
197.87 |
Myo-Inositol |
100.00 |
554.94 |
Thiamine HCl |
0.4 |
1.19 |
Appendix 5
Table A5(1): A proposed tissue culture protocol for the conservation of R. sp. from seed germplasm based on a study using R. seinghkuense.
Stage |
Step |
Day |
Process |
Description |
0 |
A |
0 |
Prepare and sterilise seeds ref. appendix 2. |
Attempt to eliminate risk of contamination |
1 |
B |
0 |
Inoculate seeds into A16 media ref. appendix 2. |
Promote germination |
1 |
C |
28 |
Maintain seedling in A16 media or re-inoculate as required. |
Shoot proliferation |
2 |
D |
56 |
Divide shoots and inoculate individually into A1 media (or A16 media to restart the cycle at step 2) |
Shoot and leaf development |
3 |
E |
84 |
Transplant into rooting media with IBA and activated charcoal per (Singh & Gurung 2, 2009) |
Root development |
4 |
F |
112 |
Transplant into sterile moss/soil substrate, protected environment, controlled humidity per (Singh & Gurung 2, 2009) |
Naturalisation 1 |
4 |
G |
140 |
Transplant into standard ericaceous soil within protected environment per (Singh & Gurung 2, 2009) |
Naturalisation 2 |
4 |
H |
168 |
Harden off to expected outdoor climate |
Hardening off â use judgement to expose gradually to change of conditions. |
|
I |
When ready |
Plant to outdoor climate |
Planting |
Appendix References
ARS, 2018. Rhododendron dalhousiae var. dalhousiae. [Online]
Available at: https://www.rhododendron.org/descriptionS_taxon.asp?ID=332
[Accessed 04 April 2018].
Flora of China, 2018. FOC Vol. 14 Page 263: Rhododendron seinghkuense. [Online]
Available at: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=242344465
[Accessed 20 February 2018].
Gibbs, D., Chamberlain, D. & Argent, G., 2011. The red list of rhododendrons. Botanic Gardens Conservation International.
Hirsutum, 2009. Rhododendron Species. [Online]
Available at: http://www.hirsutum.info/rhododendron/species/detail.php?id=674
[Accessed 28 November 2017].
Hirsutum, 2018. R. dalhousiae var dalhousiae. [Online]
Available at: http://www.hirsutum.info/rhododendron/species/detail.php?id=676
[Accessed 04 April 2018].
Hootman, S., 2004. Species profile: Rhododendron dalhousiae. [Online]
Available at: http://scholar.lib.vt.edu/ejournals/JARS/v58n2/v58n2-hootman.htm
[Accessed 04 April 2018].
McQuire, J. F. J. & Robinson, M. L. A., 2009. Pocket Guide to Rhododendron Species. 1st ed. Kew: Royal Botanic Gardens, Kew.
Paul, A., Khan, M. L., Arunachalam, A. & Arunachalam, K., 2005. Biodiversity and conservation of rhododendrons in Arunachal Pradesh in the Indo-Burma biodiversity hotspot. Current Science, 89(4), pp. 623-634.
Rhododendronforeningen, 2012. Rhododendron seinghkuense. [Online]
Available at: http://www.rhododendron.dk/seinghkuense.html
[Accessed 06 December 2017].