Pete Jorgensen
Plant Science & Horticulture
Forcing budwood and working towards a tissue culture protocol for
Pyrus cordata Desv.
Introduction
P. cordata is a rare protected species within the United Kingdom, rarely producing viable seed. Micropropagation is a useful means of conserving and propagating germplasm, but no species-specific protocol has yet been published for P. cordata. The efficacy of obtaining explants from the shoots of forced buds using warm ambient temperature and cool temperature treatment followed by a near-lethal hot water bath is investigated. Explant response to MS and MS with adjusted levels of Ca and mesos nutrients is explored. Sterilized green shoots were inoculated into four different micropropagation media (MS, MS + 2 x Ca, MS + 3 x Ca, and MS + 2x mesos) and cultivated in a grow room under 16hr fluorescent white light at 23°C (±1°C). Results show that reasonable rates of budburst (38%) can be induced in endodormic budwood within 4 weeks using warm temperature alone (>20°C). Indications are that several weeks of cool temperature treatment followed by a near-lethal hot water bath (10 minutes at 50°C) may improve rates of budburst. Results were inconclusive. Explant establishment and growth was observed across all four media tested - none produced decisively advantageous results.
Background
Pyrus cordata Desv., known as 'Plymouth Pear' following its discovery in Plymouth in 1865, has been part of Kew’s conservation program since 1876, and is one of Britain’s rarest trees (BRC, 2017; Royal Botanic Gardens, Kew, 2017). It has been the subject of passionate conservation efforts (figure 1) and the estimated wild population count is 30 - all located within Devon and Cornwall (Dodd, 2017).

Figure 1: Extract from a letter sent by Lady Sayer of Dartmoor Preservation Association to Alison Wilson, contributor to the Marine Biological Association Herbarium, 1 December 1966 (MBA, 2013).
P. cordata is listed as a species of principle importance in the Natural Environment and Rural Communities Act 2006 and before 1994 it was classed as endangered by the IUCN; it is awaiting reassessment for classification in the current IUCN red list (NERC Act, 2006; NHM, 2017; IUCN Red List, 2017). It is a protected species on schedule 8 of the Wildlife and Countryside Act (1981) and remains on the red list for Great Britain with 49 accessions listed in ex-situ germplasm collections within the UK (JNCC, 2017; Fielder et al., 2015).
The genus Pyrus has its origins in Western China from the tertiary period, making it 60 million years old (Silva et al., 2014). P. belongs to the subfamily Maloideae, the members of which generally have 17 chromosomes, including P. cordata (Postman et al., 2014). Maloideae is believed to have been hybridized from two other families within the Rosaceae – the Prunoideae (x=8) and Spiraeoideae (x=9) (Silva et al., 2014). This fusion is likely to have occurred in China - an early hotbed for the domestication of P. (Silva et al., 2014).
The debate concerning the lineage and origins of P. cordata has a history spanning at least 140 years – see appendix 1. Oliveira et al. (1999) found European wild pear, P. pyraster, shared more genetic similarities with P. cordata than P. communis (common or domestic pear, sometimes also known as European pear); however, more recent genetic studies have linked P. cordata to Asian-origin P. species with significant similarities to P. caucasica, P. spinosa and P. nivalis (figure 2), suggesting they have shared lineage from primitive stock (Royal Botanic Gardens, Kew, 2017; Zheng et al., 2014). Only two distinct genotypes of P. cordata exist in Britain (Leadlay & Jury, 2007).
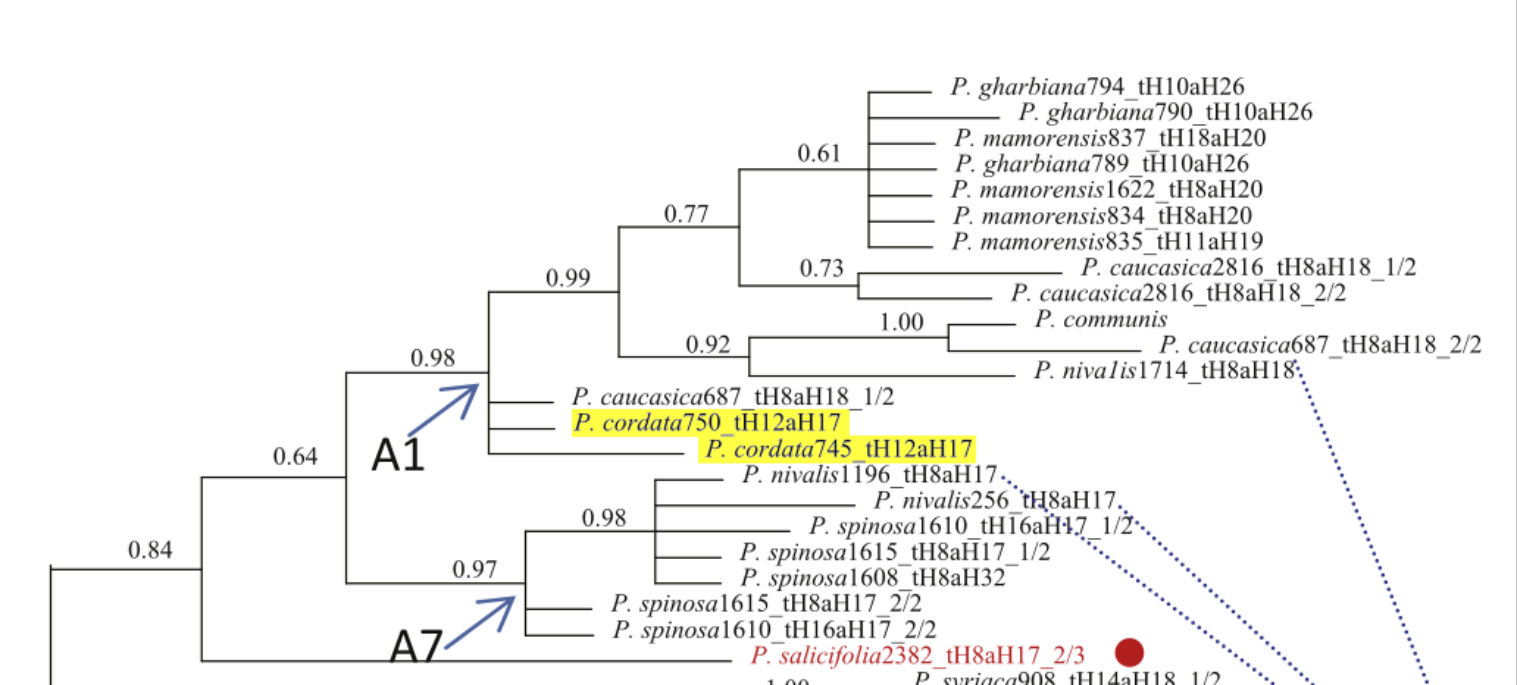
Figure 2: Bayesian consensus tree modelled from selected genetic markers showing the relationship between various Pyrus species. P. cordata is highlighted in yellow. Source: Adapted from (Zheng et al., 2014).
It is speculated that P. cordata is an archeophyte, having arrived in SW England while Britain was still physically attached to mainland Europe, however, SE England, not the SW, was attached to mainland Europe during the Mesolithic period (Dodd, 2017; Gaffney et al., 2007). Wild specimens are found in Portsmouth and Truro, both busy commercial and naval ports for centuries before the tree’s discovery, and it seems equally likely, if not more so, that germplasm arrived via ship; maybe via migrating bird. Hogs were frequently carried on board ships during the 17th and 18th centuries as food for the crew and the fruit of P. cordata is edible so may have been used as fodder (Pascual & Herrero, 2017; Somerville, 2009). Recent studies indicate that Iberian P. bourgaeana can be distributed endozoochorically via numerous animals, including wild boar, with seeds remaining viable and germinating successfully (Fedriani & Delibes, 2009). Trees found growing in the wild in the UK are located on cultivated or developed land in suburban areas - also indicating a more recent arrival. The oft-quoted status as a British native is contestable and a phytogeographical map recently published by Kew Science suggests that it is not (figure 3) (BRC, 2017; Hodder & Bullock, 1997).
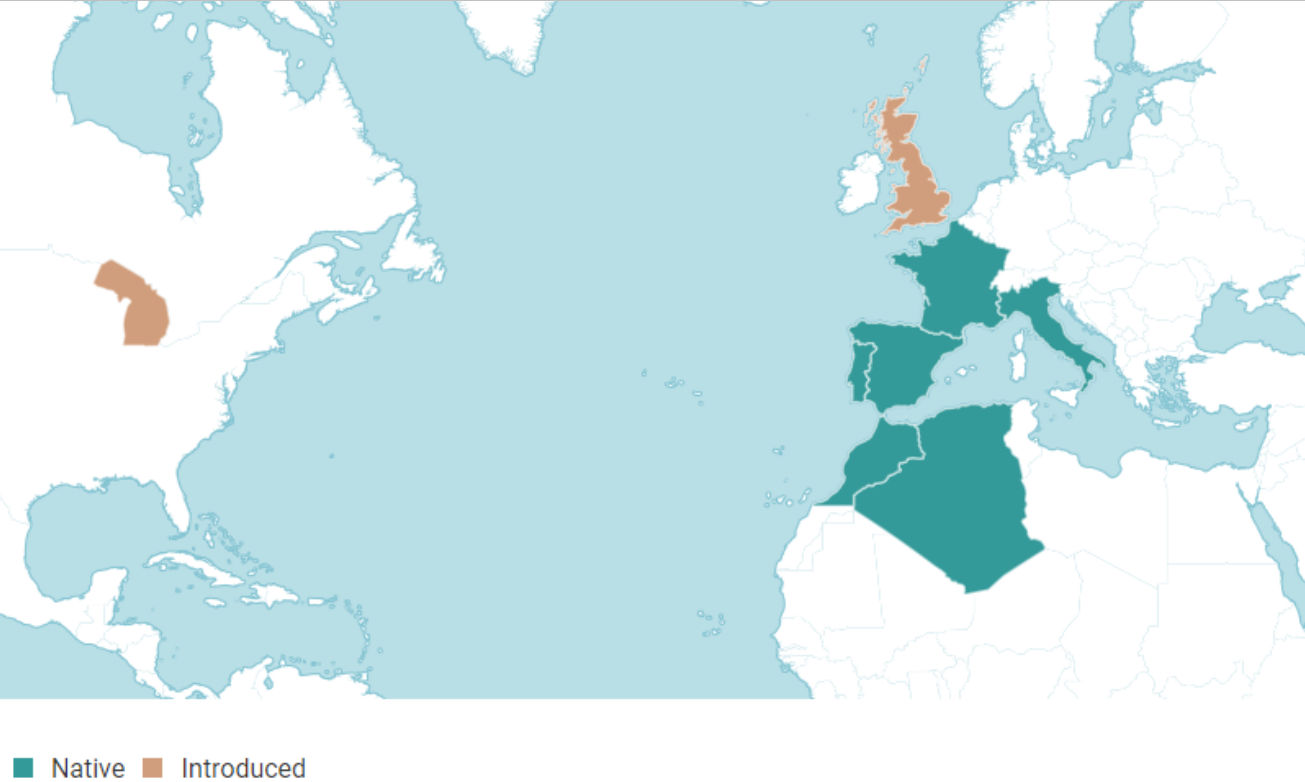
Figure 3: A phytogeographical map for P. cordata from www.plantsoftheworldonline.org, scale unavailable (Kew Science, 2017).
Pear is an important economic crop, with global annual production estimated at 27 345 930 tonnes. P. cordata is a crop wild relative and could prove invaluable for breeding germplasm with desirable traits such as pathogen resistance or climate adaptation (Hopkins, 2011; Fielder et al., 2015). Outside the UK, the species is often found in mixed deciduous woodland and inhabits large areas of NW Spain, the Iberian Peninsula - Portugal, and areas of Brittany. Some sources, such as Royal Botanic Gardens, Kew (2017) claim it is restricted to these areas but Zheng et al. (2014) document its presence in Turkey, and it is also recorded growing wild in the Carpathian Mountains, Eastern Europe.
The tree is deciduous, growing to 10m (usually shorter), and flowers in late spring, tiny fruits follow - rarely with seeds; the small number of seeds that are found are inbred or hybridized and usually not viable; it is self-incompatible and often spreads clonally through suckers (Royal Botanic Gardens, Kew, 2017; BRC, 2017). Fruits are frequently described as useless or inedible but in parts of the Pisuerga river basin, Spain, where they are known as Perujo, and the Bay of Biscay, where they are known as Basomakatza, they are eaten fresh, and used to make liquor and a cider called Pitkin (Pascual & Herrero, 2017; Menendez-Baceta et al., 2012).
Tissue culture is a valuable means of propagation when standard reproductive techniques prove difficult or unviable. Haberlandt, a German horticulturalist, is widely considered the progenitor of modern in vitro culture, having grown undifferentiated plant cells in a nutrient medium early in the 20th Century. Evidence that single plant cells can grow into entire mature plants (totipotency) led to the development of industrial techniques with the potential to create hundreds of thousands of mature plants from a tiny amount of germplasm. Much research has taken place to optimize growing media for the efficient reproduction of specific taxa. However, comparatively little research exists for P. cordata. Tissue culture protocols exist for Pyrus, but research suggests significant variation in the reaction of different pear genotypes to media with different nutrient content (Lambardi et al., 2012; Wada & Reed, 2017).
This investigation aims to discover useful factors in constructing a species-specific protocol for the micropropagation of P. cordata – specifically the establishment of healthy explants and shoot proliferation. Shoots used in micropropagation are sometimes obtained from subcultures or from mature donor plants, but forced dormant budwood has also been used (Banno et al., 1989; Thakur & Kanwar, 2008; Anirudh & Dalal, 2008). For success in forcing, dormant budwood is best harvested at the end of winter - after chilling requirements have been met (Lambardi et al., 2012). However, the need to commence this project earlier in the season required additional efforts to force buds. Therefore, initial experiments examine methods of hastening bud break before progressing to investigate establishment and growth of shoots in-vitro.
Bud Forcing
Dormancy is an essential physiological phase for many woody plants in temperate regions. Buds develop during the growing season and then enter a dormant stage with reduced metabolic activity which protects them from the winter weather and helps conserve energy while the plant is reliant on stored carbohydrates (Anderson et al., 2010). Once favourable conditions arrive buds are ready to spring into action, quickly developing photosynthetic and reproductive organs (Anderson et al., 2010).
There are three main stages of bud dormancy: paradormancy is a response to endogenous signals that originate in tissue or organs besides the bud itself - signals can be hormonal or a response to sink-source activities; endodormancy is a response to cellular signals from within the bud; ecodormancy is a response to the plant’s perception of external conditions, i.e. day length, temperature, and water availability (Tarancón et al., 2017). Internal factors involved in these processes include: phytochrome for the detection of light and red/far red ratios, and cryptochrome, inter alia, for the detection of blue light and possibly temperature (Anderson et al., 2010). When environmental change is detected, signalling proteins increase or reduce the production of other signalling molecules, such as hormones, often via the activation or inhibition of a gene (Anderson et al., 2010). The full complexity and interconnectedness of these signal transduction pathways is not yet fully understood (Martín-Fontecha et al., 2018). The type and quality of environmental variables that influence dormancy can vary considerably between species and even between varieties of the same species (Olsen, 2010; Chao et al., 2007).
A period of winter chill is usually a prerequisite for ending endodormancy and is often measured in chill units (CU) - a unit of 1 hour of exposure to temperatures within a given range. CU requirements for apple and pear are approximated to 1200 to 1500 hours at 5-7°C. However, many species have CU requirements well outside this range - even within Japanese pear species, a range from 400 (P. fauriei) to over 1800 (P. michauxii) has been found (Ryugo, 1988; Takemura et al., 2013). A search of the literature reveals no proposed CU requirement for P. cordata. Placing budwood in polyethylene bags at a controlled low temperature is suggested as an artificial method for exposing budwood to CU requirements.
Several chemical treatments have been used to force bud break in woody perennials. Thiourea was found to reduce floral bud dormancy by 8-14 days in P. pyrifolia and hydrogen cyanide (HCN) has been found to be a highly effective bud breaking agent in apple and pear (Jana & Das, 2014; Carvajal-Millan et al., 2007; Krisanapook & Subhandrabandhu, 1995). HCN is unstable and highly toxic - an unnecessary risk for use in this study.
Exogenous hormonal and chemical treatments may be unnecessary. Near-lethal temperature treatments were found to be effective in forcing budwood from Populus nigra L. harvested in November; 45°C for 2h was most successful, achieving 50% bud break, this rose to 80% for budwood collected later in the season (Wisniewski et al., 1997). Near-lethal heat treatment is believed to be most effective when budwood is in an advanced state of endodormancy, but if the ecodormant phase has commenced heat treatment may be ineffective, inhibiting bud break or killing buds entirely (Wisniewski et al., 1997). A treatment of 45°C for 4 hours is reported to have been sufficient to break floral bud endodormancy in Pyrus pyrifolia (Tamura et al., 1998). A published protocol for forcing Pyrus suggests using a 50°C hot water bath for 10 minutes (Lambardi et al., 2012). Light has been found to have no effect on the dormancy phases of P. (Heide et al., 2005).
Disinfection
Microbial contamination can be extremely detrimental to the establishment and subsequent growth of an explant. Washing plants under running water before the use of chemical treatments is recommended – particularly for woody material (Karimpour et al., 2013; Purohit, 2013; Lambardi et al., 2012). Mercuric chloride is sometimes used as an effective sterilizing agent, however, potential benefits compared to other sterilizing agents do not seem to justify the risk associated with human and plant toxicity nor the complication of disposal . It is common for short treatments with ethanol solution to form part of the sterilization process. However, this can have a desiccating effect, particularly on delicate plant tissues (Lambardi et al., 2012). Treating explant material before cutting it into segments, rather than after, can reduce contamination (Thakur & Sood, 2006).
Diluted sodium hypochlorite (NaOCl – commonly an active ingredient in household bleach) is frequently used for sterilizing plant material (Karimpour et al., 2013; Dobránszki & da Silva, 2010). The temperature at which NaOCl is applied does not seem to affect its efficacy at reducing bacterial growth and low concentrations are effective provided contact is over a longer period (>20 mins) (del Carpio-Perochena et al., 2015). The robustness of plant material needs to be considered as NaOCl is phytotoxic - CaOCl is a less harmful alternative to NaOCl, but it is less affordable and not as readily available (Trigiano & Gray, 2004; Purohit, 2013). 1% NaOCl solution (with 0.2% surfactant) for 10 minutes has been used effectively for Pyrus (Karimpour et al., 2013). Explants should be washed in sterile distilled water several times to remove phytotoxic residues following treatment with NaOCl (Karimpour et al., 2013; Lambardi et al., 2012).
Sodium Dichloroisocyanurate (NaDCC) is also a useful disinfectant and is available in sterilants such as Miton™. Its mode of action (chlorine ions) is similar to NaOCl, but it can be more effective as a microbacterial agent and less phytotoxic (Heling et al., 2001; Parkinson et al., 1996; Sarasan, et al., 2002). 15 minutes in NaDCC solution has been used as an effective disinfectant treatment for leaf explants (Leng & Keng, 2007).
Growing Media
The components of growing media for tissue culture can be separated into four main groups: sugars, gelling agents, hormones, and vitamins & minerals. Appendix 2 contains a summary of key elements and their functions in plant physiology, along with a comparison of selected media and their ingredients. Experimental tissue culture of Pyrus dates back at least as far as 1976 when sucrose was found to be a more effective sugar than sorbitol for inducing callus (Coffin et al., 1976); sorbitol is reportedly more effective than sucrose for inducing shoot proliferation in Japanese pear (Kadota et al., 2001). More recent experiments involving Pyrus tend to use growing media with sucrose - a soluble carbohydrate naturally synthesized and transported in higher plants (Thakur & Kanwar, 2008; Rehman, 2014; Trigiano & Gray, 2004).
Agar is used as a gelling agent. The amount used can affect how much support the explant has, and the rate of nutrient uptake. More agar results in a more solid gel with more support, but decreases rates of nutrient uptake. Less agar provides less support, but higher nutrient uptake (Purohit, 2013). Liquid or combined liquid and gel media requires additional support for inoculated plants, is more prone to contamination, and is less robust to movement and transportation . Gellan gum-based gelling agents such as Gelrite™ and Phytagel™ have the minor advantage of being clear rather than opaque allowing for easier observation of small changes. However, there are reports of issues with hyperhydricity (vitrification) in explants that have been grown on such formulations (Trigiano & Gray, 2004; Zimmerman et al., 1995). A concentration of 6 g/L agar can be effective but published protocols for the micropropagation of P. suggest using 7 g/L (Lambardi et al., 2012).
Hormones play a complex role in plant physiology. They affect numerous functions from the cellular level through to organ and whole plant response. In terms of tissue culture, their key roles are often discussed in simplified terms: cytokinins such as 2iP and BAP are associated with cell division, stem growth, and shoot proliferation; auxins such as IAA are associated with cell enlargement and root formation (Purohit, 2013). The balance of hormones used is crucial and will vary depending on the desired outcome, for example, establishing explants, shoot proliferation, root formation and induction of callus . Effects can be genotypically variable, and the desired balance is also dependent on how plant material responds to non-hormonal ingredients in the growing media (Purohit, 2013; George et al., 2008).
Cheng medium (Cheng, 1979) supplemented with 4.9 µM BAP was used to successfully establish P. cordata shoots in a study investigating post-cryopreservation recovery of explants (Chang & Reed, 2001). Abdollahi et al. (2006) experimented with various concentrations of BAP finding that 4.4µM was optimal for shoot regeneration in P. communis, although media was additionally supplemented with 4.9µM 2iP. Gibberellic acid has been found to inhibit shoot growth and is best excluded when establishing explants in culture (Bell & Reed, 2002; Rodriguez et al., 1991). Published protocols for Pyrus recommend the use of 4.4 µM BAP excluding other hormones such as IAA and 2iP during the establishment phase (Lambardi et al., 2012).
MS is an inorganic mineral salt media originally formulated for use with tobacco plants. It has proven to be effective for many taxa, and its mineral salt recipe forms the basis of much contemporary media. However, numerous attempts have been made to formulate a more effective media for Pyrus: Nedelcheva (1986) found QL (Quoirin & Lepoivre, 1977) induced higher rates of shoot proliferation than MS for Bartlett pear; Lloyd & McCown (1980) developed a liquid woody plant medium (WPM) that has proven to be more successful than MS with some species of Pyrus, although benefits are cultivar dependent - ½ strength MS produced more shoot growth and proliferation than WPM for several cultivars (Banno et al., 1989). QL and WPM are both low in nitrogen when compared with MS (Bell et al., 2009).
Banno et al. (1989) suggest that lower N is important for some cultivars of Japanese pear but Wada & Reed (2017) found several species of Pyrus, including pyrifolia, to be susceptible to a range of physiological defects when cultured in media with low N. Moderate concentrations of N (as ammonium and nitrate) give good shoot quality across a range of P. while optimal levels vary greatly both inter and intra species (Wada & Reed, 2017). P. cordata produces good results in shoot growth over a broad range of nitrate levels with optimal results achieved in two media - one using standard MS, the other an adjusted MS with 50% additional N; no significant difference was observed between the two treatments (Ibid.).
Wada et al. (2013) identified the critical importance of ‘mesos’ (CaCl2, MgSO4, KH2PO4) in optimizing shoot length, shoot proliferation, and plant quality, suggesting further genotype-specific studies would be useful. Simultaneously increasing Fe, ‘mesos’, and NH4NO3 in a standard MS medium can reduce hyperhydricity and necrosis (Wada & Reed, 2017). It is important to clarify that meso is also a specific term used in organic chemistry referring to compounds that have a mirror plane of symmetry (leading to the possibility of stereoisomers), but for which such isomers are achiral (can be superimposed in their mirror image) (UCLA, 2018; Khanna et al., 2006; Dewick, 2006). CaCl2, MgSO4 and KH2PO4 do have a mirror plane of symmetry, but they do not qualify as meso compounds. Meso is etymologically derived from the Greek for middle and here it is used with the simple meaning of intermediate and not in the more technical way it is used in organic chemistry (Wada et al., 2013).
Reed et al. (2013b) suggest that calcium may be the most influential meso nutrient. They refer to an experiment which observed increased levels of leaf nutrients (N, P, Zn, B, and Mg) in Bromeliads that were cultured on a media with increased Ca (Aranda-Peres et al., 2009). Divalent calcium ions (Ca2+) are a key component in many physiological functions within plants such as the alignment of the mitotic spindle during cell division, enzyme production, and the operation of signal transduction pathways (George et al., 2008). This investigation will look at the response of explants inoculated into four media: standard MS, MS + 2 x Ca, MS + 3 x Ca and MS + 2 x mesos.
Polyphenol oxidation can lead to blackening of tissues and eventually the death of the explant. Use of lower light levels and/or ascorbic acid as a pre-inoculation treatment can help prevent this (Trigiano & Gray, 2004). Plant cells produce optimal growth within a pH range of 5.3 to 5.8 (Trigiano & Gray, 2004). P. has been cultured at a pH of 5.2 and 5.8 but most frequently pH 5.7 which is recommended in published protocols (Chang & Reed, 2001; Ameri, et al., 2018; Giordani et al., 2013; Wada et al., 2013; Reed et al., 2013b; Jain & Haggman, 2007).
Methods
One-year dormant lateral branch cuttings (budsticks) from a mature P. cordata were received from Reading University on 20 November 2017 and placed in cold storage at 7°C (±1) until required. This material was used in experiments A, B, C and D. Trelissick Gardens (a National Trust property near Truro, Cornwall) supplied additional cuttings for use in experiment E. All shoots harvested were from lateral leaf buds. Data was collated and analysed in Microsoft Excel and statistical tests carried out using Minitab 18.0.
Experiment A – bud forcing (room temperature)
This was a randomised single-factor two-level (1x2) experiment designed to compare the difference a temperature treatment had on the proportion of buds that could be forced within a certain time. Sixteen budsticks were washed for 5 minutes under running tap water then soaked for 15 minutes in Domestos™ (4.5g NaOCl per 100g) solution - 20% v/v made up with sterile de-ionized water. On removal, they were trimmed into 25cm lengths using secateurs sterilized with ethanol solution. Budsticks were then rinsed thrice using sterile deionized water and dried carefully using clean paper towels. They were then arranged randomly into three groups, two of five sticks and one of six.
The group of six was placed into a clean plastic bag, sealed and stored at 7°C (±1) in the dark – for use in experiment B. The two remaining groups of 5 budsticks were placed into clean plastic containers containing 60ml aliquots of sterile deionized water fortified with plant feed (general mineral plant food – 1 x 10g sachet per litre), 1 stem per container. Each was covered with a loose fitting, clean, clear plastic bag. One group was placed on a laboratory bench near a window, the other in an outdoor unheated greenhouse. A temperature data logger was placed directly adjacent to the plants in both locations. Water levels were monitored and topped up as required; plant food solution was refreshed approximately every 14 days. A slight fungal infection (possibly botrytis) was discovered on two stems in the laboratory after 28 days. The affected stems were washed with sterile water and their tips sprayed with general-purpose fungicide (‘Fungus Clear’ – active ingredient: triticonazole). A count of total buds and of buds that had burst was made after 33 days.
Experiment B – bud forcing (near-lethal heat shock)
This was a randomized two-factor, two-level (2x2) experiment designed to discover whether a near-lethal hot water bath (HWB) was effective in increasing rates of budburst within a set timescale and whether pre-treatment with differing CUs affected the outcome. Budsticks used in this experiment were the group of 6 from cold storage described, but not used, in experiment A, plus the group of 5 from the greenhouse in experiment A that produced 0 budburst. Budsticks were sterilized using the methods described in experiment A. They were then separated randomly into a total of four groups (table 1). Groups 1 and 2 were made of 3 budsticks each from the refrigerated group of 6. Groups 3 and 4 were made of 3 and 2 budsticks each from the five from the greenhouse. Groups 1 and 3 were then treated to a HWB (sterile deionized water) at 50°C for 10 minutes using an electric Grant JB Nova 5L bath (±0.5°C). Groups 2 and 4 were simultaneously treated to a CWB (sterile deionized water) at 14.9°C for 10 minutes.
Table 1: Groups of treatments, abbreviated labels and number of stems per group for a bud forcing experiment conducted on P. cordata.

Each budstick base was trimmed 5mm using sterile secateurs and placed into a labelled plastic container containing aliquots of 60ml of deionized sterile water / plant food solution (MiracleGrow™ all-purpose plant food, NPK 6:3:6 with micronutrients). Each was covered using a clean, clear, plastic bag and sealed with an elastic band. Containers were arranged on a bench in the laboratory using a Latin square design. Plant food solution was refreshed approximately every 14 days and containers cleaned. Total number of buds per stem were counted and the number of buds per stem that had burst was recorded at 12, 19, 28 and 42 days.
Experiment C – micropropagation round 1
This was a single-factor four-level randomized design using four experimental media made up per the media preparation protocol from Purohit (2013) using ingredients listed in appendix 3, and summarized in table 2; 50ml aliquots of media were added to 250ml autoclavable plastic containers.
Table 2: Media treatment labels and summary composition for four types of gel media into which P. cordata shoots were inoculated.
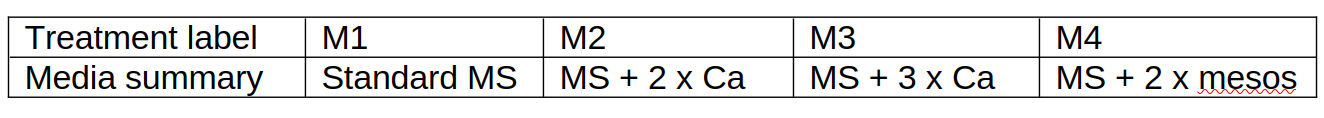
Shoots were excised from the budsticks forced in experiment A using a sterile scalpel (ethanol solution + thermal bead bath); cuts were approximately 2mm into the bark/cambium of the main holding stem, excess bark and bud scales were removed. The excised shoots were collected in a conical flask and placed in a tap water rinse for 10 minutes. Next, they were transferred to a lidded plastic container containing a magnetic stirring rod and 15% bleach solution (sterile distilled water + thin bleach, 1.5g NaOCl per 100g – giving a solution strength of 0.22% NaOCl) + 2 drops of surfactant (washing-up detergent) and stirred for 10 minutes on a magnetic stirrer. The bleach solution was drained, shoots rinsed thoroughly in sterile distilled water and then treated with a solution of 160ml sterile distilled water containing one dissolved 4g tablet of Milton™ (active ingredient: Sodium Dichloroisocyanurate 19.5% w/w) and stirred for 10 minutes on a magnetic stirrer.
Containers were then moved to the laminar flow cabinet, shoots rinsed using sterile distilled water and placed for 10 minutes into an antioxidant solution (200mg/L ascorbic acid and 150mg/L citric acid made up with sterile distilled water). Next, shoots were selected randomly and inoculated into growing media, one per pot. Containers were sealed, labelled and moved to a growth chamber - 23°C (±1) under 16h white fluorescent light - 30 µM m-2 s-1 (±1). The original design was for 10 shoots per media but due to difficulties in harvesting, losses during the forcing process, and a lower rate of budburst than had been anticipated, the numbers inoculated were M1=6, M2=6, M3=7 and M4=4 (a shoot intended for M4 was inoculated into M3 in error). Observations were made after 13, 21 and 42 days. Leaf growth was measured by counting whole leaves, half leaves and quarter leaves. Stem length was estimated using 1mm2 graph paper placed at an angle adjacent to the sealed pots. This method was preferred to using a rule against the stem as this would require removing the lid, increasing the risk of contamination; it was also preferred over the use of a graticule lens which limits measurements to a birds-eye view (the wrong plane for use with stem measurements); graph paper could be positioned at the optimal angle for each explant. Harvested shoots differed in size and quality from the outset so comparing the difference in growth from day 0 was decided upon as the best means of analysis.
By day 42 numbers had been reduced to M1=3, M2=2, M3=5 and M4=3 due to contamination. All explants showed signs of stress / necrosis by day 42. They were cleaned and subcultured, but the need to trim bases and remove necrotic leaves meant data collected after this point would not be useful.
Experiment D – micropropagation round 2
Methods used were as experiment C – shoots used were harvested from the buds forced in experiment B. Numbers inoculated were M1=5, M2=5, M3=5 and M4=9. More replicates were placed in M4 as fewer had been inoculated in experiment C; a meta-analysis may have been possible to assimilate the two sets of results, but observations could not be matched temporally, so results were not comparable enough to be combined. The number of replicates available for analysis by day 28 had been reduced to M1=1, M2=3, M3=5 and M4=9 due to contamination or necrotic death.
Experiment E – micropropagation round 3
Due to reduced replicate numbers in experiments C and D, additional budstick material was sourced from Trelissick Gardens near Truro, Cornwall, England to conduct a repeat experiment. Buds were forced quickly by treating them to room temperature for 2 weeks, but just days before harvesting shoots became necrotic and began to die. Buds that could be used were inoculated. Unfortunately, >80% became contaminated, and the experiment was abandoned.
Results
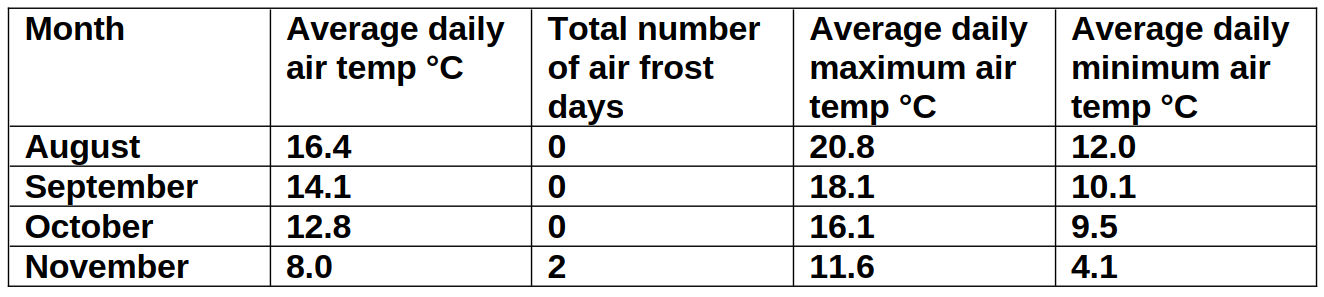
Four months of climate data prior to harvesting budwood from Reading University (table 3), used in experiments A, B, C and D, suggests exposure to negligible CU. None from August to October, and of the 17 x 24 hours measured in November, just over half (204) are likely to have been <9°C.
Table 3: Ambient air temperature P. cordata budwood donor plants were exposed to between 1st August 2017 and 17th November 2017. Source: (University of Reading, 2017).
Experiment A - bud forcing (room temperature)
H0 = There will be no difference in percentage rates of bud break in P. cordata stems subject to two different long-term ambient temperature treatments.
Maximum and minimum temperatures in the laboratory were 26.9°C and 14.1°C; 788 hours > 16°C (figure 4). Mean day temperature (7am-6.59pm) was 21.8°C and the mean night temperature was 20°C (7pm-6.59am). Temperature within the greenhouse was more varied with a maximum of 32.4°C and minimum of -0.2°C; 271 hours<6°C, 506 hours<9°C, 684<12°C, 43 hours>16°C (figure 5). Mean day temperature was 10°C and the mean night temperature was 6°C. The first five days of data (6th to 10th December) were unavailable due to a technical error.
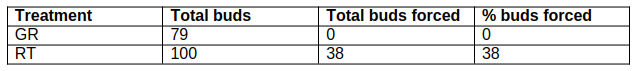
38% of buds stored at room temperature burst (n=100) compared with 0 from the outdoor unheated greenhouse (n=79) – per table 4. A Fisher’s exact test gave p<0.001 - a very high level of confidence that H0 can be rejected.
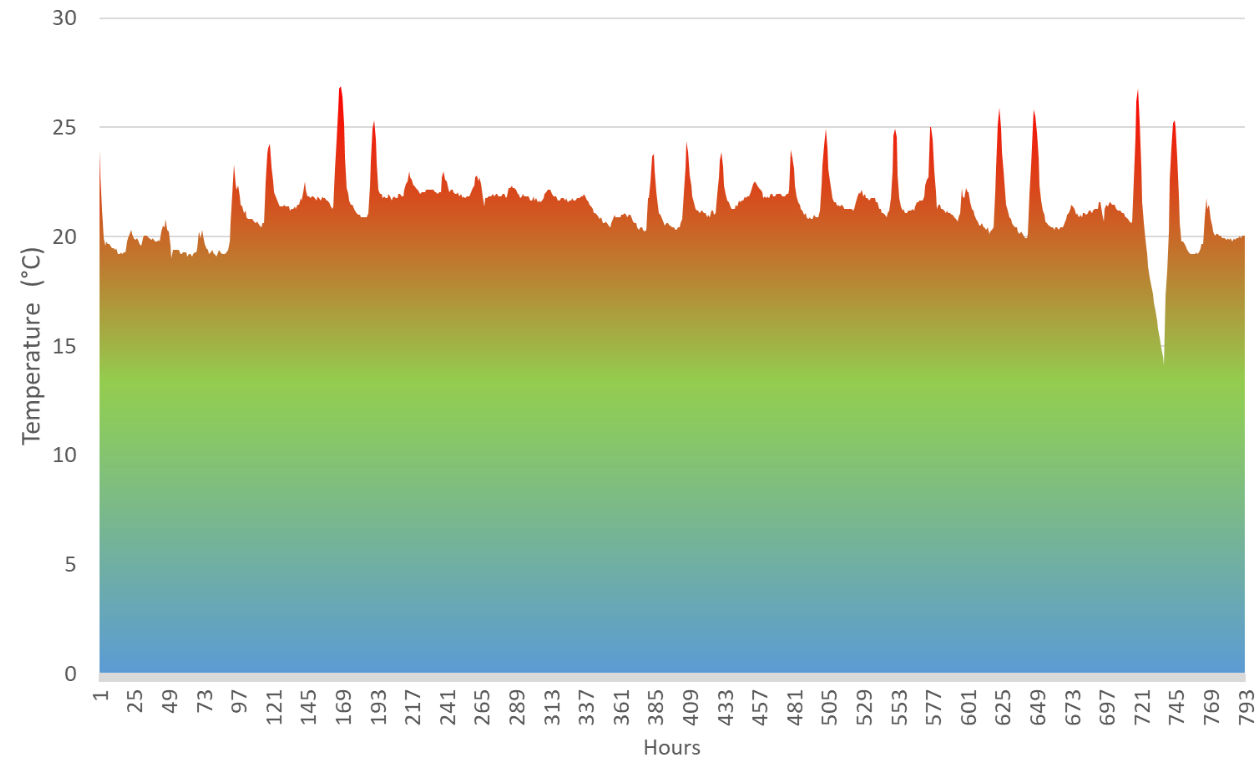
Figure 4: Forcing P. cordata budwood at room temperature: hourly recorded temperature from 3pm, 11th December 2017, to 3pm, 8th January 2017, 793 data points.
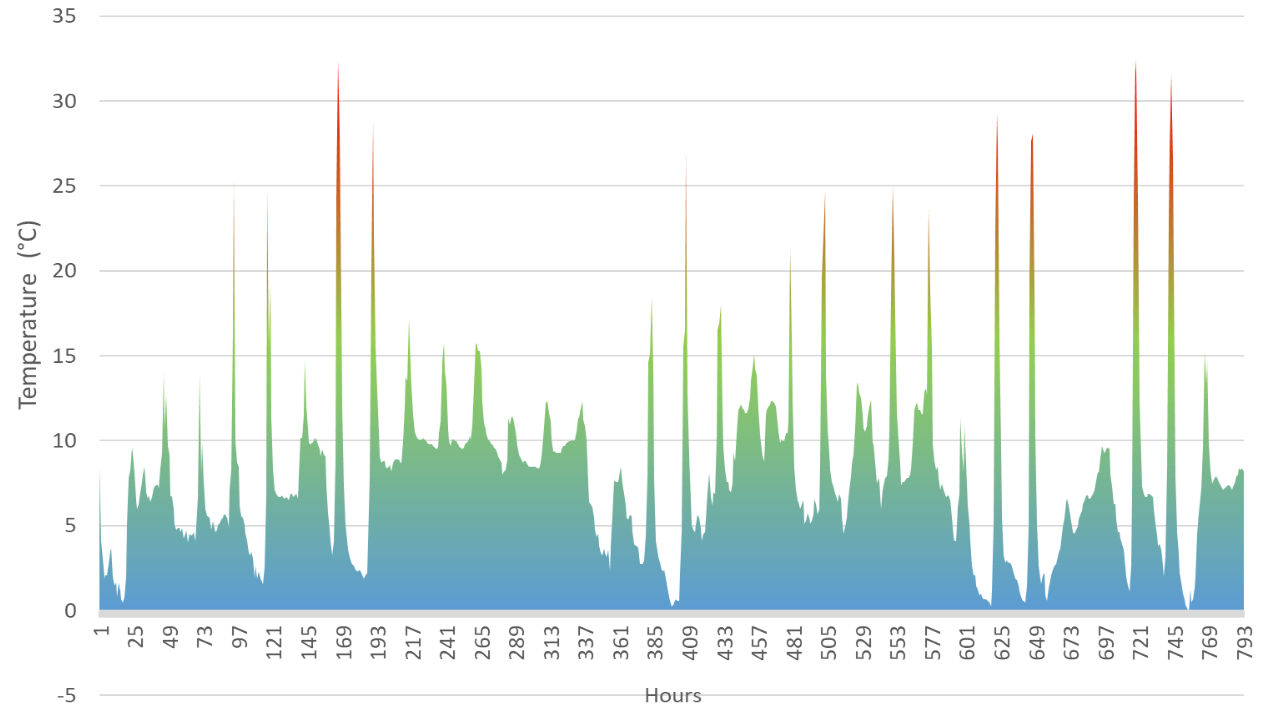
Figure 5: Forcing P. cordata budwood in an unheated greenhouse: hourly recorded temperature from 3pm, 11th December 2017, to 3pm, 8th January 2017, 793 data points.
Table 4: % of P. cordata buds that had burst after 33 days following two different ambient temperature treatments. GR = outdoor greenhouse, RT = room temperature.
Experiment B - bud forcing (near-lethal heat shock)
H0 = There will be no difference in rates of budburst for P. cordata subject to different CU treatments followed by either a hot or cold-water bath by day 42.
The Mean percentage budburst for each group of stems over time (figure 6) suggests an initial trend by day 19 - budburst on stems from the greenhouse subject to both HWB and CWB but little activity from those that had been refrigerated. By day 42 the group that had been refrigerated and then treated with a HWB (FH) had a much higher percentage of bud break – the error bar is large as all FH shoots came from one stem. Some counts (GH + GC) fell due to buds dying.
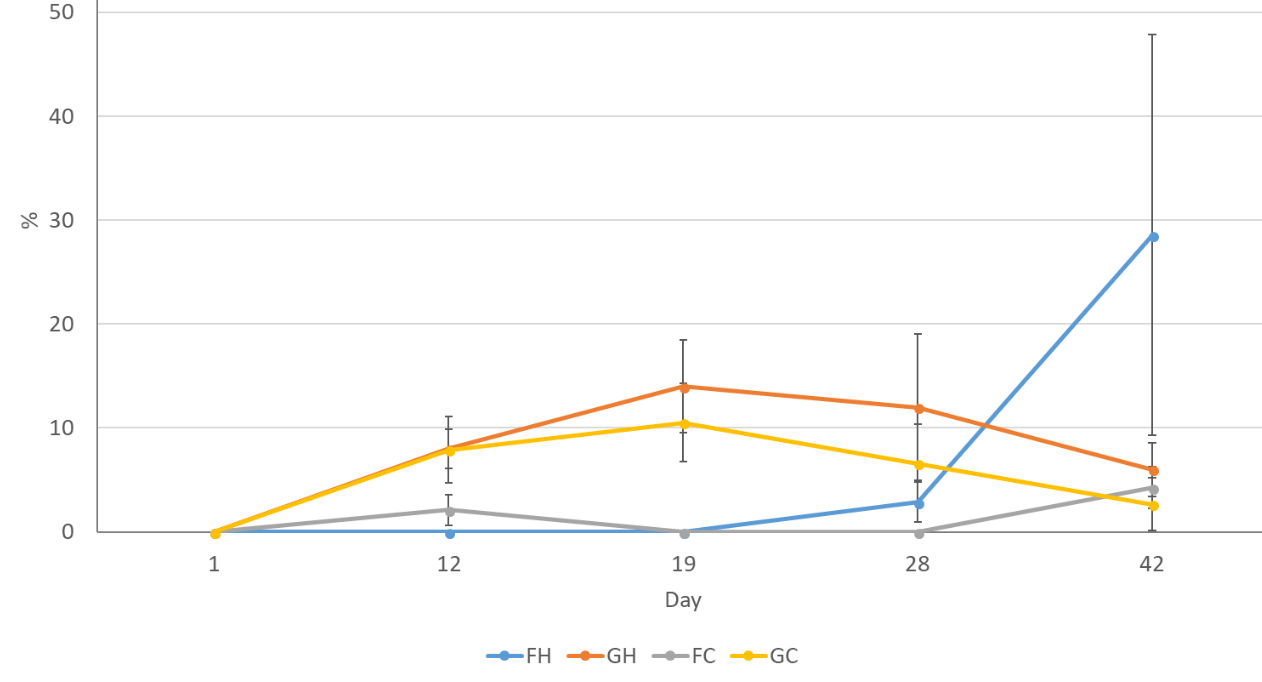
Figure 6: % budburst over time for P. cordata subject to four different treatments: FH=cool temperature (7°C ±1) followed by hot water bath (HWB) at 50°C for 10 minutes, GH=ambient unheated greenhouse followed by HWB. FC= cool temperature (7°C ±1) followed by a cool water bath (CWB) 14.9°C for 10 minutes, GC=ambient unheated greenhouse followed by CWB. Error bars show standard error of the mean.
Confidence intervals are a more statistically useful way of analysing results than p values (Colquhoun, 2014). An interval plot based on 95% confidence intervals shows we cannot reject the null hypothesis (figure 7).
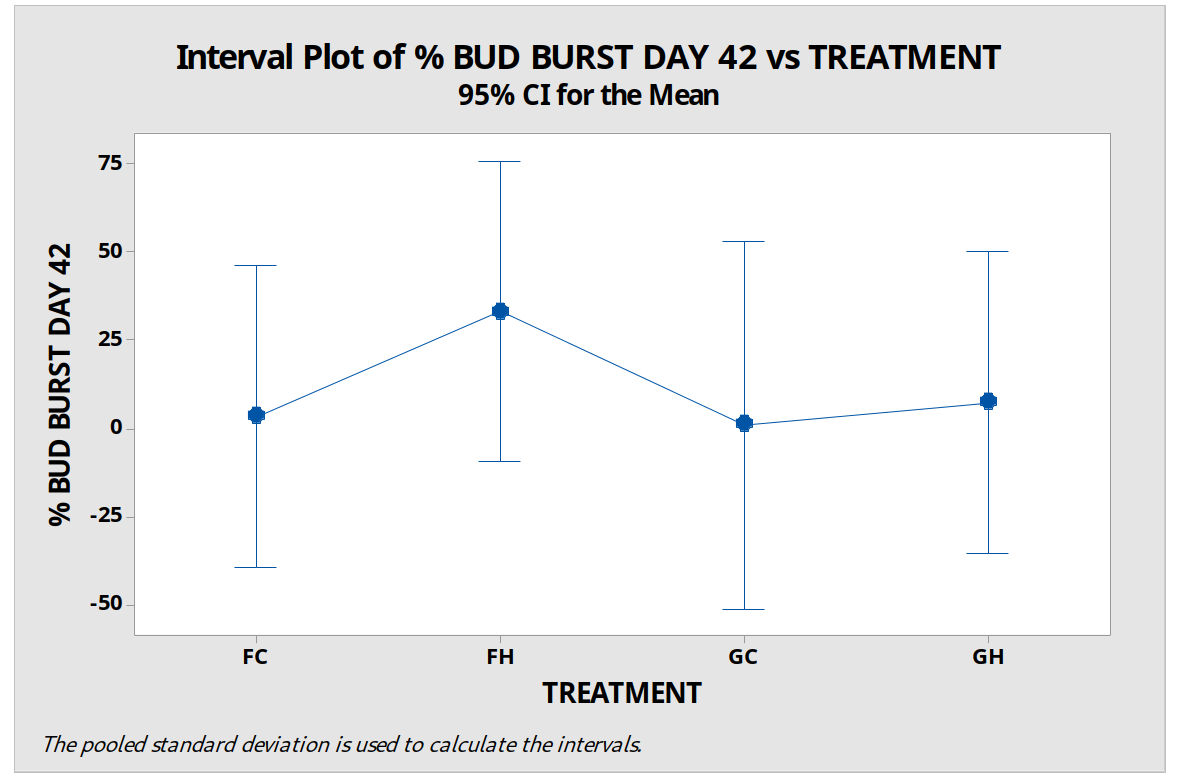
Figure 7: % budburst on day 42 for four treatment groups of P. cordata woody stems with variation bars based on 95% confidence intervals. FH=cool temperature (7°C ±1) followed by hot water bath (HWB) at 50°C for 10 minutes, GH=ambient unheated greenhouse followed by HWB. FC= cool temperature (7°C ±1) followed by a cool water bath (CWB) 14.9°C for 10 minutes, GC=ambient unheated greenhouse followed by CWB. Error bars show standard error of the mean.
Experiment C – micropropagation round 1
a) Leaf growth: H0 = There will be no difference in leaf growth by day 42 between four groups of P. cordata subject to treatment with four different growing media: M1, M2, M3 and M4.
New leaf development was evident for explants grown in all four media at each observation point up to day 42 - figure 8. Error bars show the standard error of the mean and these results suggest a difference between M1 and M3 on day 13, M1 and M2/M3 on day 21 and M1 and M2/M3/4 on day 42. However, an interval plot, based on a 95% confidence interval (figure 9) shows that we cannot reject the null hypothesis.
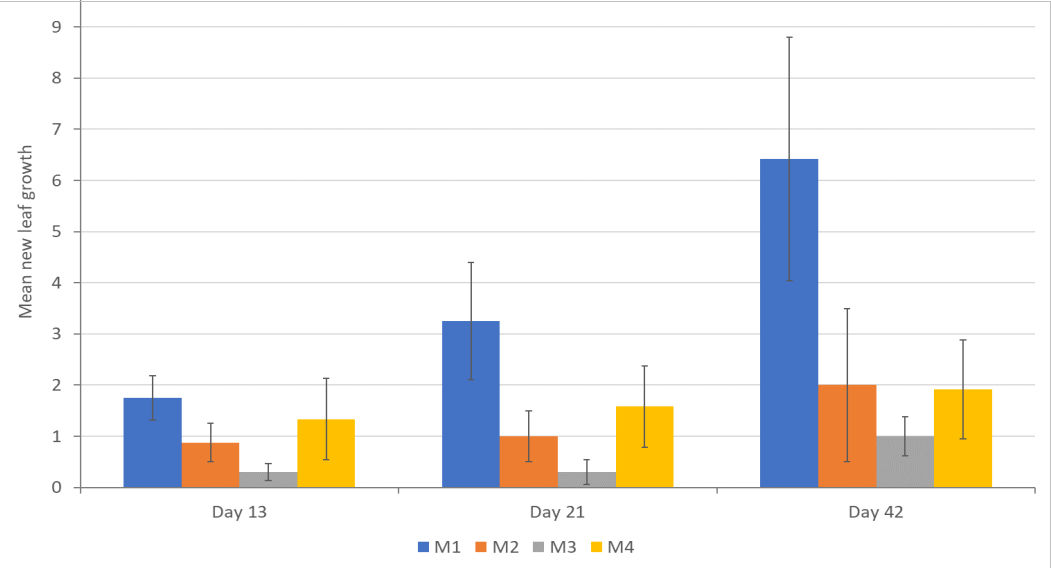
Figure 8: Mean new leaf growth on day 13, 21 and 42 for P. cordata explants grown in four different test media. M1=standard MS media, M2=MS + 2 x Ca, M3=MS + 3 x Ca, M4=MS+2 x mesos.
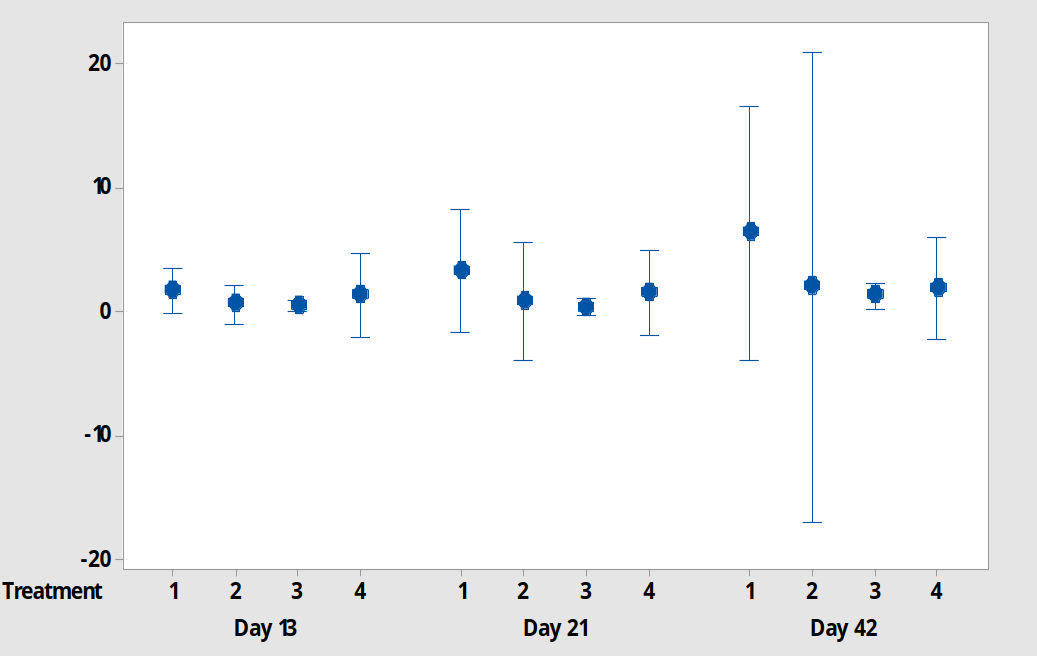
Figure 9: A 95% confidence interval plot for new leaf growth on P. cordata explants grown in 4 different test media on day 13, 21 and 42. 1=standard MS media, 2=MS + 2 x Ca, 3=MS + 3 x Ca, 4=MS+2 x mesos.
b) Stem growth H0 = there will be no difference in mean stem growth between groups of explants grown for 42 days in four different treatment media: M1, M2, M3 and M4.
There is no difference in increased stem length between treatment groups (figure 10) and data does not support a rejection of H0.
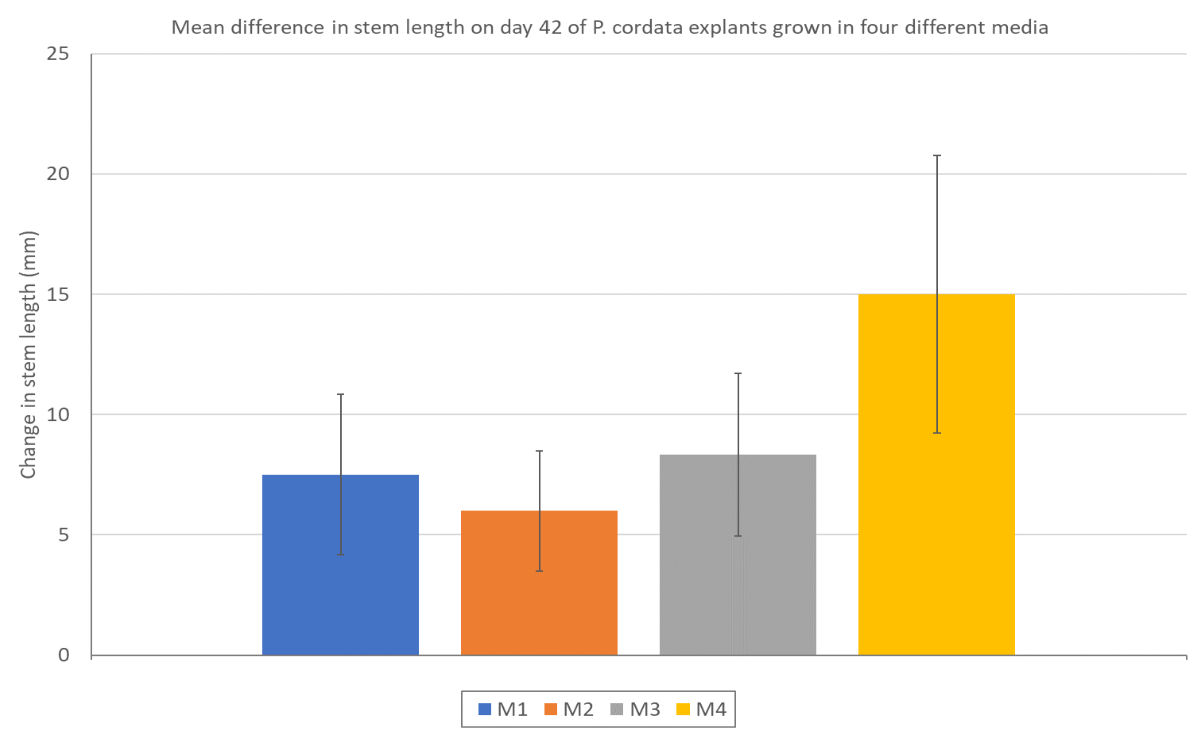
Figure 10: Change in stem length between day 0 and day 42 of P. cordata explants grown in four different treatment media. M1=standard MS media, M2=MS+ 2 x Ca, M3=MS+3 x Ca, M4=MS+2 x mesos. Error bars show the standard error of the mean.
The photographic record of an example explant (figure 11) shows that by day 13 there was browning of leaves, particularly lower ones, however, the plant continued to grow and develop healthy new shoots. By day 42 (figure 11, day 42a) the lower part of the plant was significantly chlorotic/necrotic; it was trimmed, cleaned and subcultured (figure 11, day 42b). Within 14 days of being subcultured (figure 11, day 57) the explant displayed similar signs of disease which seemed to be spreading more quickly and with a more severe effect. This pattern is representative of the majority of explants. Some budsticks used showed signs of stress/disease prior to harvesting shoots, but a decision was made to continue with the experiment due to the difficulty of obtaining fresh budwood.

Figure 11: An example of the development of a P. cordata explant treated with M1 (standard MS media) between day 0 and day 57. On day 42 the explant was cleaned, dead plant material removed and subcultured in the same media – day 42b.
Experiment D - micropropagation round 2
a) Leaf growth: H0 = There will be no difference in leaf growth between four groups of explants treated with different growth media, M1, M2, M3 and M4.
Analysis by interval plot for a confidence level of 95% - figure 12 - confirms that we can have no confidence in any differences between the groups. Group 1 was reduced to 1 explant so there are no error bars.
b) Stem length: H0 = There will be no difference in the mean increase in stem length between different groups of P. cordata explants treated to different growing media.
Due to the short length of the experiment and complications with contamination, results were not particularly useful. A 95% confidence interval plot for mean increase in stem length at day 28 shows that there is no justification for rejecting the null hypothesis (figure 13).
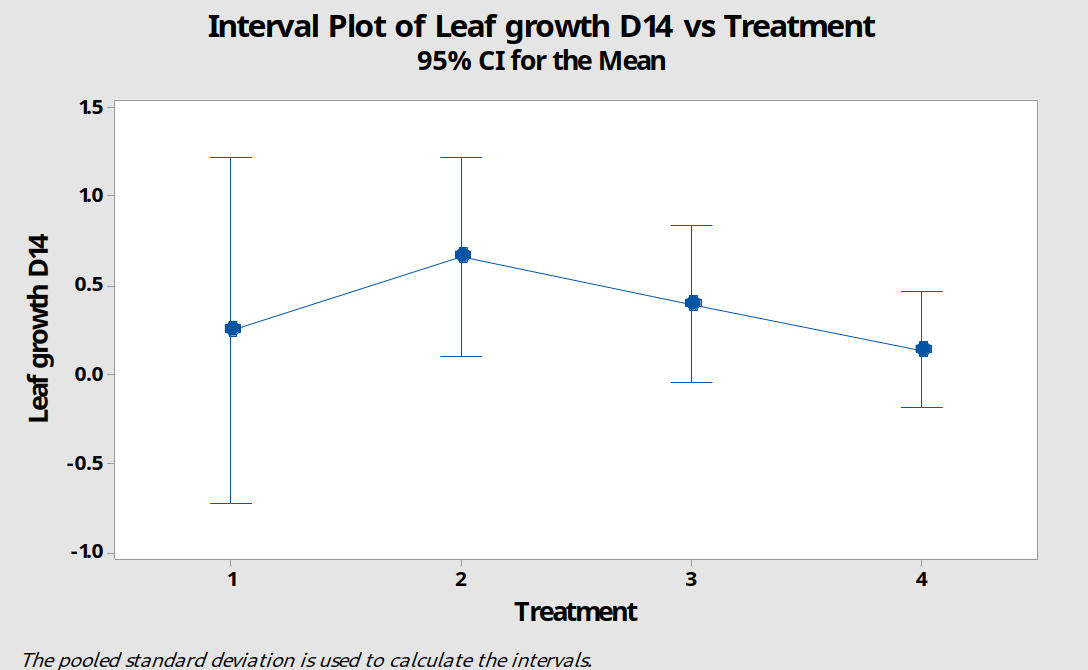
Figure 12: A 95% confidence interval plot comparing new leaf growth by day 14 for four groups of P. cordata explants subject to treatment with different growing media. M1=standard MS media, M2=MS+2 x Ca, M3=MS+3 x Ca, M4=MS+2 x mesos.
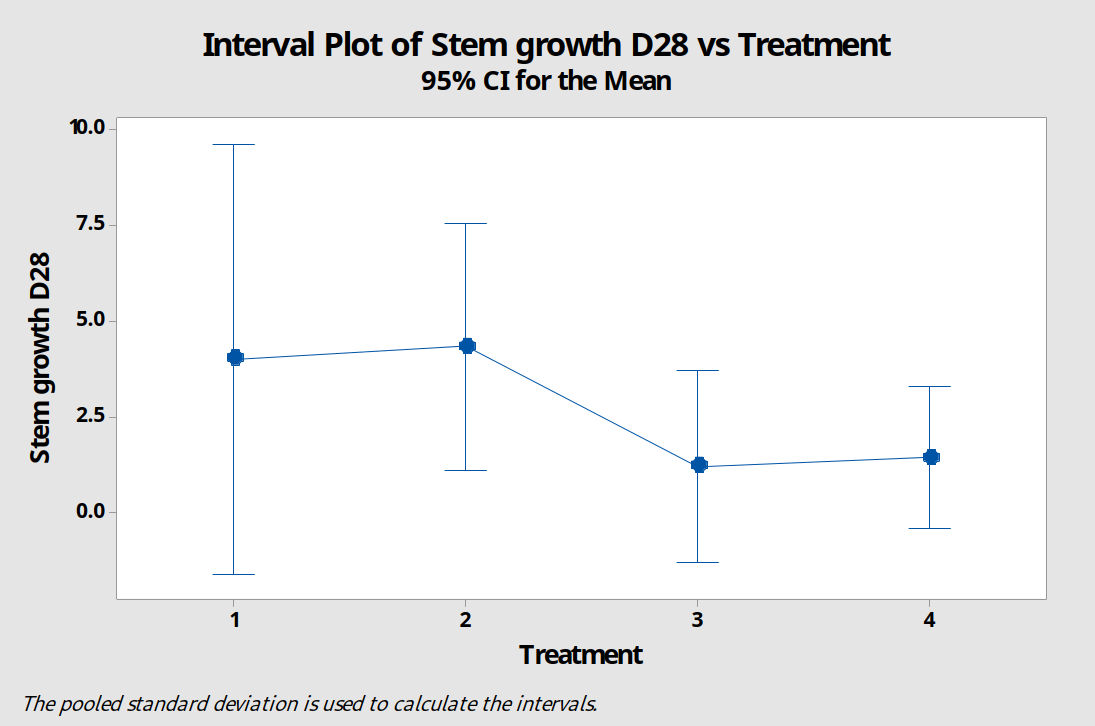
Figure 13: A 95% confidence interval plot comparing stem growth of P. cordata explants treated with four different growing media. M1=standard MS media, M2=MS+3 x Ca, M3=MS+3 x Ca, M4=MS+2 x mesos.
Discussion
Bud Forcing
Results from experiments A and B show that P. cordata can be forced using a simple ambient temperature treatment of approximately 20°C. Budwood was collected in November. It is estimated they were subject to approximately 204 CU<9°C pre-harvest, followed by a further 384 CU<9°C in cold storage, total 588, prior to commencement of experiment A. While the exact CU requirements for P. cordata were not known at the time of this experiment, exposure appeared sufficient to induce endodormancy but not post-vernal ecodormancy. P. pyrifolia was found to have different rates of leaf budburst when treated with warmth depending on the length of vernal exposure – 10% of buds burst in November compared to over 70% after mid-January (Takemura et al., 2011). Higher winter temperatures increase the hours of exposure required. For example, in P. pyrifolia 'Nakai' 750h exposure to 0-6°C was sufficient to end endodormancy whereas 1160h were required at 9°C; 12°C was completely ineffective (Sugiura & Honjo, 1997). The rate of 38% observed here for P. cordata suggests that additional chilling – perhaps a total of 1000 CU @ < 9°C may have yielded higher rates of bud break; exposure to lower temperatures <6°C for a shorter period may be equally effective. Budwood used in experiment E was subject to almost a full winter season and although not subject to experimental measurement, it had very high rates of budburst within 2 weeks at ambient room temperature.
Experiment B was inconclusive, but results suggest a controlled cool temperature treatment followed by a 10-minute hot water bath at 50°C may be a useful bud forcing treatment, this concurs with Lambardi et al. (2012). Near-lethal heat causes a metabolic shock inducing altered physiological function such as a sudden increase in the release of sugars – suspected to be causally linked with the cessation of endodormancy (Ben Mohamed et al., 2014). Other potential mechanisms involve proteins: heat may directly denature, or induce a metabolic breakdown of, proteins involved in circadian clock regulation; alternatively heat shock proteins may be invoked which then awaken metabolic processes within the cell (Wisniewski et al., 1996; Wisniewski et al., 1997).
Contamination
Field grown woody perennials are known to be difficult to effectively decontaminate - the more delicate the plant material the more difficult this becomes (Trigiano & Gray, 2004). Some shoots harvested for use in this experiment were tiny, and contamination did occur, but many explants were cultured without obvious contamination. By contrast, experiment E suffered from widespread and severe contamination. This budwood came from a different source and began to show signs of disease prior to inoculation. The sterilization process may have failed, or endogenous contaminants may have been present when harvested.
Choudhary et al. (2015) excised almond buds, and forced them in-vitro, after removing the outer scales. This method could make sterilization more effective because budwood could be scrubbed directly before excision. However, P. cordata buds are very small and delicate. Removing outer scales would leave minimal explant material, increasing the chances of handling damage, and contamination during inoculation.
Necrosis
Physiological dysfunction such as shoot tip necrosis, callus formation and hyperhydricity is frequently observed in the micropropagation of pear (Reed et al., 2013a). The browning and death of P. explants during the establishment phase has been attributed to phenol exudates (Banno et al., 1989). Anti-oxidant treatment (ascorbic acid) was used here, but Banno et al. (1989) experimented with liquid cultures, activated charcoal, and ascorbic acid. They did not eliminate necrosis. However, subcultures every 2-3 weeks were found to be an effective means of overcoming it, and the problem usually dissipated by the fourth transplant. Time and resource constraints prevented this sort of protocol being followed here, but other potential explanations for the development of necrosis should also be considered.
Calcium deficiency is associated with curling and blackening around leaf margins, often followed by death (George et al., 2008). This seems an unlikely cause here; calcium levels were high and other experiments have been conducted using similar calcium levels without reports of such symptoms (Wada et al., 2013). Ions compete for uptake - increased Ca2+ could inhibit the uptake of Mg2+ (George et al., 2008), but the pattern of necrosis observed was not typical of Mg deficiency. Again, Wada et al. (2013) reported no such issues.
Surface sterilization does not deal with internalized pathogens. The Rosaceae family, to which P. cordata belongs, is susceptible to Erwinia amylovora – fire blight – currently causing considerable damage to tree populations throughout the UK (BRC, 2017). Although budwood was cleaned, and had protective wrapping during the forcing procedure, it was exposed on numerous occasions to air within the laboratory. The facilities were kept immaculately clean, but they are subject to footfall from a large cohort of students who have been exposed to a range of outdoor environments. Windows are sometimes opened for ventilation, and plant matter regularly brought in for lectures and coursework. Erwinia amylovora is endophytic and can remain present within plant tissues for some time without external symptoms (Miller & Schroth, 1972). Oozing is a common symptom associated with fire blight, but its absence does not exclude the presence of infection (Vanneste, 2008). There are striking similarities between symptoms of fire blight and necrotic symptoms on explants and on budwood – figure 14.

Figure 14: Left - a canker-like darkening of stem and side-branch with a wilted, curled leaf discovered on budwood of P. cordata during bud forcing experiment B. Right – the necrotic death of an explant with contamination emanating from the point of inoculation.
Media
In contrast with Wada et al. (2013) no evidence was found to suggest that MS media with increased Ca or 2 x mesos enhanced the success of establishing explants nor led to increased leaf shoot proliferation when compared to standard MS. However, repeat experiments with more replicates would be required to form a confident opinion about this. Buds used in experiments C and D were subject to different treatments for forcing. It is possible that this could influence later development of the shoots. Future experiments could focus on developing this area of knowledge.
Conclusion
P. cordata is rare in England, but its status as a native archeophyte is questionable. Populations of P. cordata are found in various other locations, and it is not clear whether it is rare or threatened in these other habitats. Decoding the exact genotypic differences between the species found in Britain and those elsewhere may help identify how unique the specimens located in Britain are. Meanwhile, it would seem sensible to continue efforts to protect and conserve P. cordata germplasm in England.
Ex-situ conservation is possible through micropropagation using forced budwood and shoots can be obtained within 4 weeks using warm temperatures alone (20°C); artificial chilling followed by a HWB of 50°C for 10 minutes may help force budwood has had insufficient CUs but collecting budwood after it has experienced sufficient CUs will yield optimal results. Explants were cultured and survived on all four media tested (MS, MS + 2 x Ca, MS + 3 x Ca, and MS + 2 x mesos). Nothing was discovered to suggest there would be any benefit in altering the Pyrus micropropagation protocol published by Lambardi et al. (2012).
Establishing explants and proliferating shoots without experiencing necrosis, disease, or contamination is difficult. Without understanding the cause of the necrosis observed, recommending specific actions to avoid it is speculative. Ensuring donor plants are disease-free, and thorough sterilization of harvested plant material, is essential. A protected, sterile environment for bud forcing would be beneficial. Frequent subcultures – minimum every 14 days – may help produce explants free from necrosis by the fourth iteration.
Running the experiment with more replicates, and using a preliminary liquid culture explant contaminant detection protocol, as described by Lambardi et al. (2012), is suggested. If contaminants are detected, identifying them will help develop a procedure for avoidance, or treatment. Prolonged experiments, including rooting and hardening off, would be required for a complete propagation protocol that results in physiologically complete young plants. Further experiments comparing the in vitro response of P. cordata in MS to other media, such as WPM and LP, would help refine the tissue culture protocol.
References
Abdollahi, H., Muleo, R. & Rugini, E., 2006. Optimisation of regeneration and maintenance of morphogenic callus in pear (Pyrus communis L.) by simple and double regeneration techniques. Scientia horticulturae, 108(4), pp. 352-358.
Ameri, A., Davarynejad, G. H., Moshtaghi, N. & Tehranifar, A., 2018. Polyethylene glycol and chilling overcome Somatic embryogenesis obstacle in Pyrus communis. Scientia Horticulturae, Volume 227, pp. 57-64.
Anderson, J. V., Horvath, D. P., Chao, W. S. & Foley, M. E., 2010. Dormancy and resistance in harsh environments. In: S. Hohman, ed. Bud dormancy in perennial plants: a mechanism for survival. Berlin: Springer, pp. 69-90.
Anirudh, T. & Dalal, R. P. S., 2008. Micropropagation of pear (Pyrus spp.): a review. Agricultural Reviews, 29(4), pp. 260-270.
Aranda-Peres, A. N., Peres, L. E. P., Higashi, E. N. & Martinelli, A. P., 2009. Adjustment of mineral elements in the culture medium for the micropropagation of three Vriesea bromeliads from the Brazilian Atlantic Forest: the importance of calcium. HortScience, 44(1), pp. 106-112.
Banno, K., Yoshida, K., Hayashi, S. & Tanabe, K., 1989. In vitro propagation of Japanese pear cultivars. Journal of the Japanese Society for Horticultural Science, 58(1), pp. 37-42.
Bell, R. L. & Reed, B. M., 2002. In vitro tissue culture of pear: Advances in techniques for micropropagation and germplasm preservation. Acta Hort , Issue 596, pp. 412-418.
Bell, R. L., Srinivasan, C. & Lomberk, D., 2009. Effect of nutrient media on axillary shoot proliferation and preconditioning for adventitious shoot regeneration of pears. In Vitro Cellular & Developmental Biology-Plant, 45(6), p. 708.
Ben Mohamed, H., Zrig, A., Geuns, J. & Khemira, H., 2014. Near-lethal heat treatment induced metabolic changes associated with endodormancy release of superior seedless grapevine cv.('Vitis vinifera'L.) buds. Australian Journal of Crop Science, 8(4), pp. 486-494.
Bojnanský, V. & Fargašová, A., 2007. Atlas of seeds and fruits of Central and East-European flora: the Carpathian Mountains region. 1st ed. Berlin: Springer Science & Business Media.
BRC, 2017. RDB Species Accounts: Pyrus cordata Desv. (Rosaceae). [Online]
Available at: https://www.brc.ac.uk/plantatlas/plant/pyrus-cordata
[Accessed 10 November 2017].
Carvajal-Millan, E. et al., 2007. Respiratory response of apple buds treated with bud breaking agents. Thermochimicha Acta, Volume 457, pp. 109-112.
Chang, Y. & Reed, B. M., 2001. Preculture Conditions Influence Cold Hardiness and Regrowth of Pyrus cordata Shoot Tips After Cryopreservation. HortScience, 36(7), pp. 1329-1333.
Chao, W. S., Foley, M. E., Horvath, D. P. & Anderson, J. V., 2007. Signals regulating dormancy in vegetative buds. International Journal of Plant Developmental Biology, 1(1), pp. 49-56.
Cheng, T. Y., 1978. Clonal propagation of woody plant species through tissue culture techniques [Fruit, ornamentals]. In: Combined Proceedings International Plant Propagators' Society. s.l.:s.n.
Choudhary, R., Chaudhury, R., Malik, S. K. & Sharma, K. C., 2015. An efficient regeneration and rapid micropropagation protocol for Almond using dormant axillary buds as explants. Indian Journal of Experimental Biology, 53(July), pp. 462-467.
Coffin, R., Taper, C. D. & Chong, C., 1976. Sorbitol and sucrose as carbon source for callus culture of some species of the Rosaceae. Canadian Journal of Botany, 54(7), pp. 547-551.
Colquhoun, D., 2014. An investigation of the false discovery rate and the misinterpretation of p-values. Royal Society open science, 1(3), p. 140216.
del Carpio-Perochena, A. et al., 2015. Effect of temperature, concentration and contact time of sodium hypochlorite on the treatment and revitalization of oral biofilms. Journal of dental research, dental clinics, dental prospects, 9(4), p. 209.
Dewick, P., 2006. Essentials of Organic Chemistry. 1st ed. Chichester: Wiley.
Dobránszki, J. & da Silva, J. A. T., 2010. Micropropagation of apple - a review. Biotechnology Advances, 28(4), pp. 462-488.
Dodd, R., 2017. The history of Plymouth's rarest tree revealed. [Online]
Available at: http://www.plymouthherald.co.uk/news/local-news/history-plymouths-rarest-tree-revealed-301329
[Accessed 09 29 2017].
FAO, 2017. FAOSTAT. [Online]
Available at: http://www.fao.org/faostat/en/#data/RP
[Accessed 20 November 2017].
Fedriani, J. M. & Delibes, M., 2009. Seed dispersal in the Iberian pear, Pyrus bourgaeana: a role for infrequent mutualists. Ecoscience, 16(3), pp. 311-321.
Fielder, H. et al., 2015. Enhancing the conservation of crop wild relatives in England. PloS one, 10(6), p. p.e0130804.
Gaffney, V., Thomson, K. & Fitch, S., 2007. Mapping Doggerland: The Mesolithic Landscapes of the Southern North Sea. 1st ed. Oxford: Archaeopress.
Gardener's Chronicle, 1874. Origin of Fruit Trees. In: Gardener's Chronicle Volume 1: January to June. London: Bradbury,Agnew & Co., p. 86.
George, E. F., Hall, M. A. & De Klerk, G. J., 2008. The components of plant tissue culture media I: macro-and micro-nutrients. In: Plant propagation by tissue culture. Netherlands: Springer, pp. 65-113.
Giordani, E., Naval, M. & Benelli, C., 2013. In Vitro Propagation of Persimmon (Diospyros kaki Thunb.).. Methods in molecular biology, 10(1007), pp. 89-98.
Heide, O. M. & Prestrud, A. K., 2005. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree physiology, 25(1), pp. 109-114.
Heling, I. et al., 2001. Bactericidal and cytotoxic effects of sodium hypochlorite and sodium dichloroisocyanurate solutions in vitro. Journal of endodontics, 27(4), pp. 278-280.
Hodder, K. H. & Bullock, J. M., 1997. Translocations of native species in the UK: implications for biodiversity. Journal of Applied Ecology, Volume 34, pp. 547-565.
Hopkins, J. a. M. N., 2011. Crop Wild Relatives: Plant conservation for food security. Natural England Research Reports, Volume 37.
IUCN Red List, 2017. The IUCN Red List of Threatened Species. [Online]
Available at: http://www.iucnredlist.org/
[Accessed 11 October 2017].
Jain, S. M. & Haggman, H., 2007. Protocols for Micropropagation of Woody Trees and Fruits. 1st ed. Dordrecht: Springer.
Jana, B. R. & Das, B., 2014. Effect of dormancy breaking chemicals on flowering, fruit set and quality in Asian pear (Pyrus pyrifolia L.). African Journal of Agricultural Research, 9(1), pp. 56-60.
JNCC, 2017. Taxon Designations. [Online]
Available at: http://jncc.defra.gov.uk/files/Taxon_designations_20170718.zip
[Accessed 17 10 2017].
Kadota, M., Imizu, K. & Hirano, T., 2001. Double-phase in vitro culture using sorbitol increases shoot proliferation and reduces hyperhydricity in Japanese pear. Scientia horticulturae, 89(3), pp. 207-215.
Karimpour, S., Davarynejad, G. H., Bagheri, A. & Tehranifar, A., 2013. In Vitro Establishment and Clonal Propagation of Sebri Pear Cultivar. Journal of Agricultural Science and Technology, Volume 15, pp. 1209-1217.
Kew Science, 2017. Pyrus cordata Desv.. [Online]
Available at: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:730782-1
[Accessed 11 May 2018].
Khanna, S. K., Verma, N. K. & Kapila, B., 2006. Objective Questions in Organic Chemistry for Competitive Examinations. 2nd ed. New-Delhi: Golden Bells.
Krisanapook, K. & Subhandrabandhu, 1995. Effect of some practices and hydrogen cyanide on bud break of 'Shinseiki' pear. Acta Horticulturae, Issue 395, pp. 149-152.
Lambardi, M., Ozudogru, E. A. & Jain, S. M., 2012. Protocols for Micropropagation of Selected Economically-Important Horticultural Plants. 1st ed. Totowa: Humana Press.
Leadlay, E. & Jury, S., 2007. Taxonomy and Plant Conservation. 1sr ed. Cambridge: Cambridge University Press.
Leng, C. E. & Keng, C. L., 2007. Induction of Somatic Embryogenic Callus From the Leaves of Pereskia grandifolia. Biotechnology, 6(1), pp. 45-48.
Lloyd, G. & McCown, B., 1980. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Combined Proceedings, International Plant Propagators' Society, 30(5), pp. 421-427.
Martín-Fontecha, E. S., Tarancón, C. & Cubas, P., 2018. To grow or not to grow, a power-saving program induced in dormant buds. Current opinion in plant biology, Volume 41, pp. 102-109.
MBA, 2013. An unusual trio of Plymouth plants. Falmouth: The Marine Biological Association of the UK.
Melke, A., 2015. The physiology of chilling temperature requirements for dormancy release and bud-break in temperate fruit trees grown at mild winter tropical climate.. Journal of Plant Studies, 4(2), pp. 110-156.
Menendez-Baceta, G. et al., 2012. Wild edible plants traditionally gathered in Gorbeialdea (Biscay, Basque Country). Genetic Resources and Crop Evolution, 59(7), pp. 1329-1347.
Miller, T. D. & Schroth, M. N., 1972. Monitoring the Epiphytic Population of Erwinia amylovora. Phytopathology, Volume 62, pp. 1175-1182.
Murashige, T. & Skoog, F., 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum, 15(3), pp. 473-497.
Nedelcheva, S., 1986. Effect of inorganic components of the nutrient medium on in vitro propagation of pears. Genetics Selection Evolution, Volume 19, pp. 404-406.
NERC Act, 2006. Natural Environment and Rural Communities Act 2006. [Online]
Available at: https://www.legislation.gov.uk/ukpga/2006/16
NHM, 2017. Pyrus cordata Desv. - Plymouth Pear. [Online]
Available at: http://www.nhm.ac.uk/our-science/data/uk-species/species/pyrus_cordata.html
[Accessed 13 November 2017].
Oliveira, C. M. et al., 1999. Molecular typing ofPyrusbased on RAPD markers. Scientia Horticulturae, Volume 79, pp. 163-174.
Olsen, J. E., 2010. Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant molecular biology, 73(1-2), pp. 37-47.
Parkinson, M., Prendergast, M. & Sayegh, A. J., 1996. Sterilisation of explants and cultures with sodium dichloroisocyanurate. Plant Growth Regulation, 20(1), pp. 61-66.
Pascual, J. C. & Herrero, B., 2017. Wild food plants gathered in the upper Pisuerga river basin, Palencia, Spain. Botany Letters, 164(3), pp. 263-272.
Postman, J., Bassil, N. & Bell, R., 2014. Ploidy of USDA World Pear Germplasm Collection Determined by Flow Cytometry. XII International Pear Symposium, Volume 1094, pp. 75-81.
Purohit, S. D., 2013. Introduction to Plant Cell, Tissue and Organ Culture. 1st ed. Delhi: PHI Learning Private Limited.
Quoirin, M. & Lepoivre, P., 1977. Improved Media for In Vitro Culture of Prunus Sp.. Acta Horticulturae, Volume 78, pp. 437-442.
Reed, B. M., Wada, S., DeNoma, J. & Niedz, R. P., 2013a. Mineral nutrition influences physiological responses of pear in vitro. In Vitro Cellular & Developmental Biology-Plant, 49(6), pp. 699-709.
Reed, B. M., Wada, S., DeNoma, J. & Niedz, R. P., 2013b. Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cellular & Developmental Biology-Plant, 49(3), pp. 343-355.
Rehman, H. E., 2014. In vitro establishment and proliferation of Pyrus species. International Journal of Interdisciplinary Research , 1(8), pp. 2349-5480.
Rodriguez, R., Diaz-Sala, C., Cuozzo, L. & Ancora, G., 1991. Pear in vitro propagation using a double-phase culture system. HortScience, 26(1), pp. 62-64.
Royal Botanic Gardens, Kew, 2017. Plymouth Pear Pyrus cordata. [Online]
Available at: https://adoptaseed.kew.org/support-kew/adopt-a-seed/pyrus-cordata.htm
[Accessed 29 09 2017].
Ryugo, K., 1988. Fruit culture: its science and art. 1st ed. New York: John Wiley and Sons.
Sarasan, V., Ramsay, M. & Roberts, A., 2002. In vitro germination and induction of direct somatic embryogenesis in'Bottle Palm'[Hyophorbe lagenicaulis (L. Bailey) HE Moore], a critically endangered Mauritian palm. Plant Cell Reports, 20(12), pp. 1107-1111.
Scientia Horticulturae, 2018. Scientia Horticulturae: Author Information Pack. [Online]
Available at: https://www.elsevier.com/journals/scientia-horticulturae/0304-4238?generatepdf=true
[Accessed 20 April 2018].
Silva, G. J., Souza, T. M., Barbieri, R. L. & de Oliveira, A. C., 2014. Origin, domestication, and dispersing of pear (Pyrus spp.). Advances in Agriculture, p. Article ID 541097.
Somerville, B., 2009. Animal Invaders: Wild Boar. 1st ed. Michigan: Cherry Lake Publishing.
Sugiura, T. & Honjo, H., 1997. The effects of temperature on endodormancy completion in Japanese pear (Pyrus pyrifolia Nakai) and modeling the relationship. Journal of Agricultural Meteorology, 53(4), pp. 285-290.
Takemura, Y., Kuroki, K., Matsumoto, K. & Tamura, F., 2013. Cultivar and areal differences in the breaking period of bud endodormancy in pear plants. Scientia Horticulturae, Issue 154, pp. 20-24.
Takemura, Y. et al., 2011. Chilling induces bud endodormancy in Japanese pear ‘Gold Nijisseiki’. Horticultural Research, 10(1), pp. 87-92.
Tamura, F., Tanabe, K., Itai, A. & Tanaka, H., 1998. Protein Changes in the Flower Buds of Japanese Pear during Breaking of Dormancy by Chilling or High Temperature Treatment. Journal of the American Society for Horicultural Science, 4(123), pp. 532-536.
Tarancón, C. et al., 2017. A conserved carbon starvation response underlies bud dormancy in woody and Herbaceous Species. Frontiers in plant science, 8(May), p. Article 788.
Thakur, A. & Kanwar, J. S., 2008. Micropropagation of 'Wild pear' Pyrus pyrifolia (Burm F.) Nakai. II. Induction of Rooting. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 36(2), pp. 104-111.
Thakur, R. & Sood, A., 2006. An efficient method for explant sterilization for reduced contamination. Plant cell, tissue and organ culture, 84(3), pp. 369-371.
Trigiano, R. N. & Gray, D. J., 2004. Plant development and biotechnology. 1st ed. London: CRC press.
UCLA, 2018. Index of harding ec_tutorials:Meso compounds. [Online]
Available at: http://www.chem.ucla.edu/~harding/ec_tutorials/tutorial17.pdf
[Accessed 22 April 2018].
University of Reading, 2017. University of Reading Dept of Meteorology. [Online]
Available at: http://www.met.reading.ac.uk/weatherdata/Reading_monthly_summaries.html
[Accessed 19 November 2017].
Vanneste, J., 2008. Datasheet: Erwinia amylovora (fireblight). [Online]
Available at: https://www.cabi.org/isc/datasheet/21908
[Accessed 28 April 2018].
Wada, S., Niedz, R. P., DeNoma, J. & Reed, B. M., 2013. Mesos components (CaCl2, MgSO4, KH2PO4) are critical for improving pear micropropagation. In Vitro Cellular & Developmental Biology-Plant, 49(3), pp. 356-65.
Wada, S. & Reed, B. M., 2017. Trends in culture medium nitrogen requirements for in vitro shoot growth of diverse pear germplasm. Acta Horticulturae, 3(1155), pp. 29-36.
Wildlife and Countryside Act (1981) Available at https://www.legislation.gov.uk/ukpga/1981/69/schedule/8.
Wisniewski, M. et al., 1996. Sublethal stress and bud dormancy in woody plants. In: G. A. Lang, ed. Plant Dormancy: Physiology, Biochemistry, and Molecular Biology. Oxon: CAB International, pp. 201-210.
Wisniewski, M., Sauter, J., Fuchigami, L. & Stepien, V., 1997. Effects of near-lethal heat stress on bud break, heat-shock proteins and ubiquitin in dormant poplar (Populus nigra Charkowiensis× P. nigra incrassata). Tree physiology, 17(7), pp. 453-460.
Zheng, X. et al., 2014. Phylogeny and Evolutionary histories of Pyrus L. revealed by phylogenetic trees and networks based on data from multiple DNA sequences.. Molecular Phylogenetics and Evolutio, Issue 80, pp. 54-65.
Zimmerman, R. H., Bhardwaj, S. V. & Fordham, I. M., 1995. Use of starch-gelled medium for tissue culture of some fruit crops. Plant cell, Tissue and Organ culture, 43(3), pp. 207-213.
Appendices
Extract from the Gardener’s Chronicle 1874 illustrating the debate concerning the taxonomical categorisation and origin of various Pyrus species (Gardener's Chronicle, 1874)

Appendix 2
How plants use nutritional elements and a comparison of the composition of selected growing media.
Table A1: Essential plant macronutrients, micronutrients and vitamins and examples of their uses. Synthesised from (Purohit, 2013; George et al., 2008; *Pezzarossa et al., 2012; *Bojorquez-Quintal, 2017; *Deshmukh et al., 2017). References in main reference list except * below.
Essential Macronutrients |
|
Nitrogen |
Chlorophyll, alkaloids, nucleic acid, nucleotides, some hormones, amino acids and co-enzymes. |
Phosphorous |
Energy systems, nucleic acids, sugars, phospholipids. |
Potassium |
Cell division. Synthesis of: chlorophyll, carbohydrates, amino acids and proteins. Enzyme activation. |
Calcium |
Cell walls, movement of carbohydrates and amino acids. |
Magnesium |
Chlorophyll, enzyme activation. |
Sulphur |
Amino acids, co-enzyme A |
Iron |
Chlorophyll synthesis, energy conversion â photosynthesis and respiration, cytochromes. |
Essential Micronutrients |
|
Boron |
Transport of sugars, influences Ca2+ usage. |
Chlorine |
Stimulation of photosynthesis |
Copper |
Energy conversion, activation of enzymes. |
Manganese |
Chloroplast membrane, activation of enzymes. |
Molybdenum |
Nitrogen conversion/fixation |
Nickel |
Activation of enzymes. |
Zinc |
Enzymes, formation of chlorophyll and hormones |
Beneficial elements |
|
Aluminium |
Growth stimulant, nutrient uptake, alleviates abiotic stress. |
Cobalt |
Nitrogen fixation |
Selenium |
Alleviate abiotic stress, antioxidant |
Silicon |
Increased abiotic stress, alleviates high salinity, pathogen defence |
Sodium |
Can fulfil some of the roles of potassium |
Iodine |
Ingredient for some amino acids |
Vitamins |
|
Thiamine |
Co-enzymes |
Pyridoxine |
Co-enzymes |
Nicotinic acid |
Co-enzymes |
Myoinositol |
Synthesis of cell walls and membranes |
Table A2: A comparison of the composition of MS DKW WPM and LP growing media. Synthesized from (*Nas & Read, 2004; *Plantigen, 2018; Jain & Haggman, 2007). References in main reference list except * below.
|
MS |
DKW |
WPM |
LP |
Macrominerals mg/L |
||||
NH4NO3 |
1650 |
1416 |
400 |
400 |
Ca(NO3)2.4H2O |
- |
1960 |
556 |
578.92 |
CaCl2.2H2O |
440 |
147 |
96 |
- |
MgSO4.7H2O |
370 |
740 |
370 |
175.79 |
KNO3 |
1900 |
- |
- |
1800 |
KH2PO4 |
170 |
259 |
170 |
270 |
K2SO4 |
- |
1560 |
990 |
- |
Microminerals mg/L |
||||
H3BO3 |
6.2 |
4.8 |
6.2 |
6.2 |
CuSO4.5H2O |
0.025 |
0.25 |
0.25 |
0.025 |
MnSO4.H2O |
16.9 |
33.5 |
22.3 |
0.76 |
Na2MoO4.2H2O |
0.25 |
0.39 |
0.25 |
0.25 |
ZnSO4.7H2O |
8.6 |
- |
8.6 |
8.6 |
Zn(NO3)2.6H2O |
- |
17 |
- |
- |
FeSO4.7H2O |
27.8 |
33.4 |
27.8 |
- |
Na2.EDTA |
37.3 |
44.7 |
37.3 |
37.3 |
KI |
0.83 |
- |
- |
0.08 |
CoCl2.6H2O |
0.025 |
- |
- |
0.025 |
Vitamins mg/L |
||||
Thiamine (B1) |
0.1 |
2.0 |
1.0 |
0.4 |
Nicotinic acid (B3) |
0.5 |
2.0 |
0.5 |
- |
Pyrodoxine (B6) |
0.5 |
- |
0.5 |
- |
Amino acids mg/L |
||||
Glycine |
2.0 |
2.0 |
2.0 |
- |
Vitamins |
||||
Myo-inositol |
100 |
1000 |
100 |
1 |
Sugar |
||||
Sucrose (g/L) |
30 |
30 |
20 |
|
Appendix 2 References
Bojórquez-Quintal, E., Escalante-Magaña, C., Echevarría-Machado, I. & Martínez-Estévez, M., 2017. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Frontiers in plant science, Volume 8, p. 1767.Deshmukh, R. K., Ma, J. F. & Belanger, R., 2017. Role of Silicon in Plants. Frontiers in plant science, Volume 8, p. 1858.
Nas, M. N. & Read, P. E., 2004. A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Scientia Horticulturae, 101(1-2), pp. 189-200.
Pezzarossa, B., Remorini, D., Gentile, M. L. & R, M., 2012. Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. Journal of the Science of Food and Agriculture, 92(4), pp. 781-6.
Plantigen, 2018. Plantigen:Quoirin & Lepoivre Medium. [Online] Available at: http://www.himedialabs.com/TD/PT056.pdf [Accessed 20 April 2018].
Appendix 3
Table A3: Formulae for experimental media used for the inoculation of P. cordata explants, key adjustments being investigated are shown in bold type.
Stock code |
Ingredient(s) |
g/L stock solution |
Test media Components per Litre |
|||
M1 |
M2 |
M3 |
M4 |
|||
A |
NH4NO3 |
82.5 |
20mL |
20mL |
20mL |
20mL |
B |
KNO3 |
95 |
20mL |
20mL |
20mL |
20mL |
C |
CaCl22H2O |
44 |
10mL |
20mL |
30mL |
20mL |
D |
KH2PO4 |
17 |
10mL |
10mL |
10mL |
20mL |
E |
MgSO47H2O |
37 |
10mL |
10mL |
10mL |
20mL |
F |
MnSO4H2O CuSO45H2O ZnSO47H2O
|
1.66 0.0025 0.860 |
10mL |
10mL |
10mL |
10mL |
G |
KI CoCl26H2O H3BO3 Na2MoO42H2O
|
0.083 0.0025 0.620 0.025 |
10mL |
10mL |
10mL |
10mL |
H |
FeSO47H2O EDTA-Na2
|
2.78 3.724 |
10mL |
10mL |
10mL |
10mL |
n/a |
Thiamine (10ml/L) |
|
25mL |
25mL |
25mL |
25mL |
n/a |
Inositol |
|
250mg |
250mg |
250mg |
250mg |
n/a |
Sucrose |
|
30g |
30g |
30g |
30g |
n/a |
N6 Benzyladenine (4.4um sol)
|
|
10ml |
10ml |
10ml |
10ml |
n/a |
pH adjusted using NaOH |
|
5.7 |
5.7 |
5.7 |
5.7 |
n/a |
Agar |
|
7 g/L |
7 g/L |
7 g/L |
7 g/L |